
Value Delivered
The prior art enabled the client to file an opposition against a threatening patent, resulting in the client’s entry into the market with their generic drug.
Problems Solved
Invalidating dosage regimen patents is challenging due to their complex claims with narrow limitations. In this specific case, the challenge arose in linking a disease’s surrogate marker to the dosing nuances of a supporting therapy— a connection completely missing in traditional patent searches.
Even unconventional explorations like conference posters, textbooks, and videos offered no breakthrough. Adding to the complexity, the need for equivalence among multiple claimed factors and precise frequency of adjustments posed an extra layer of difficulty.
Solution Offered
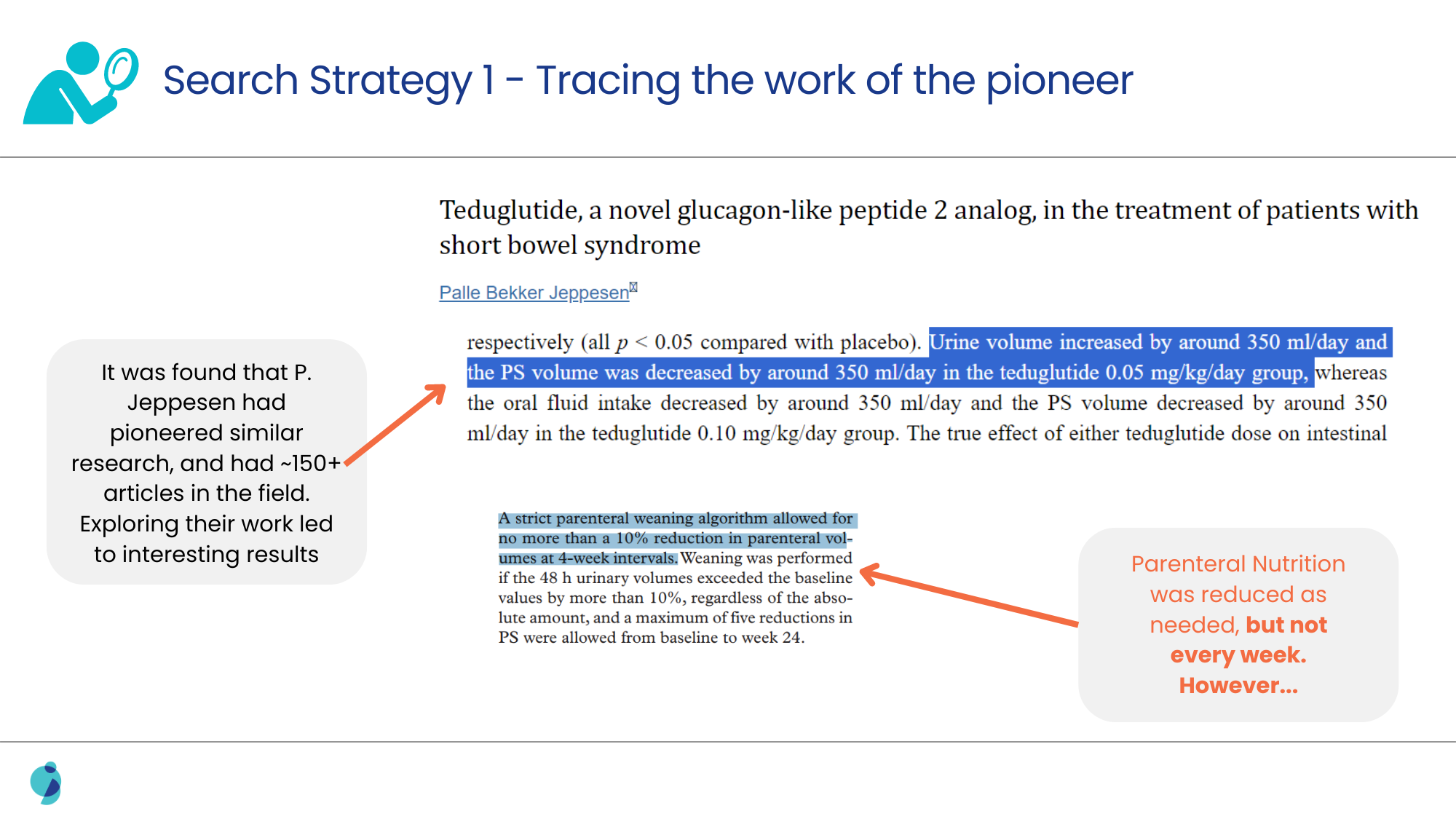
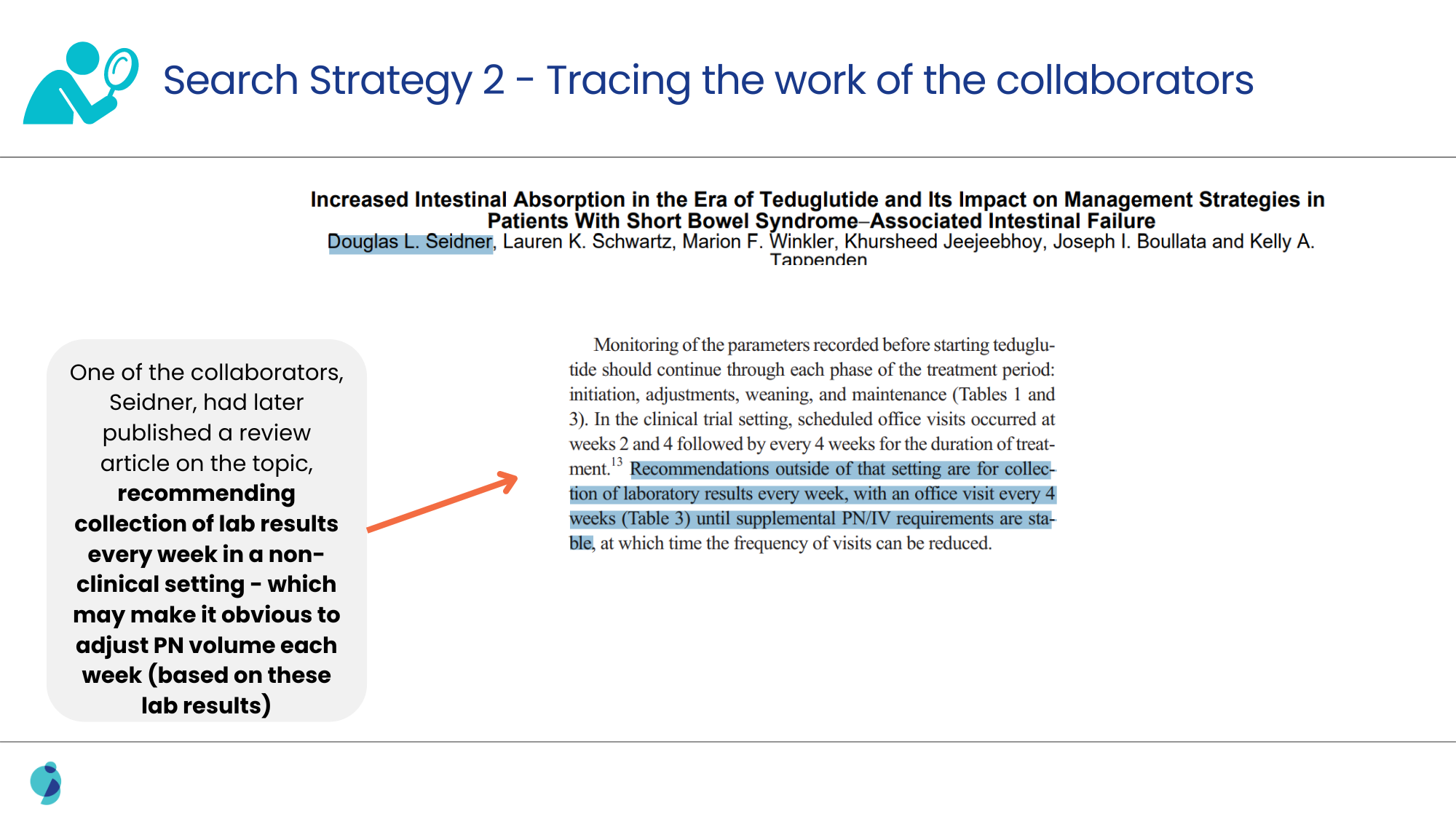
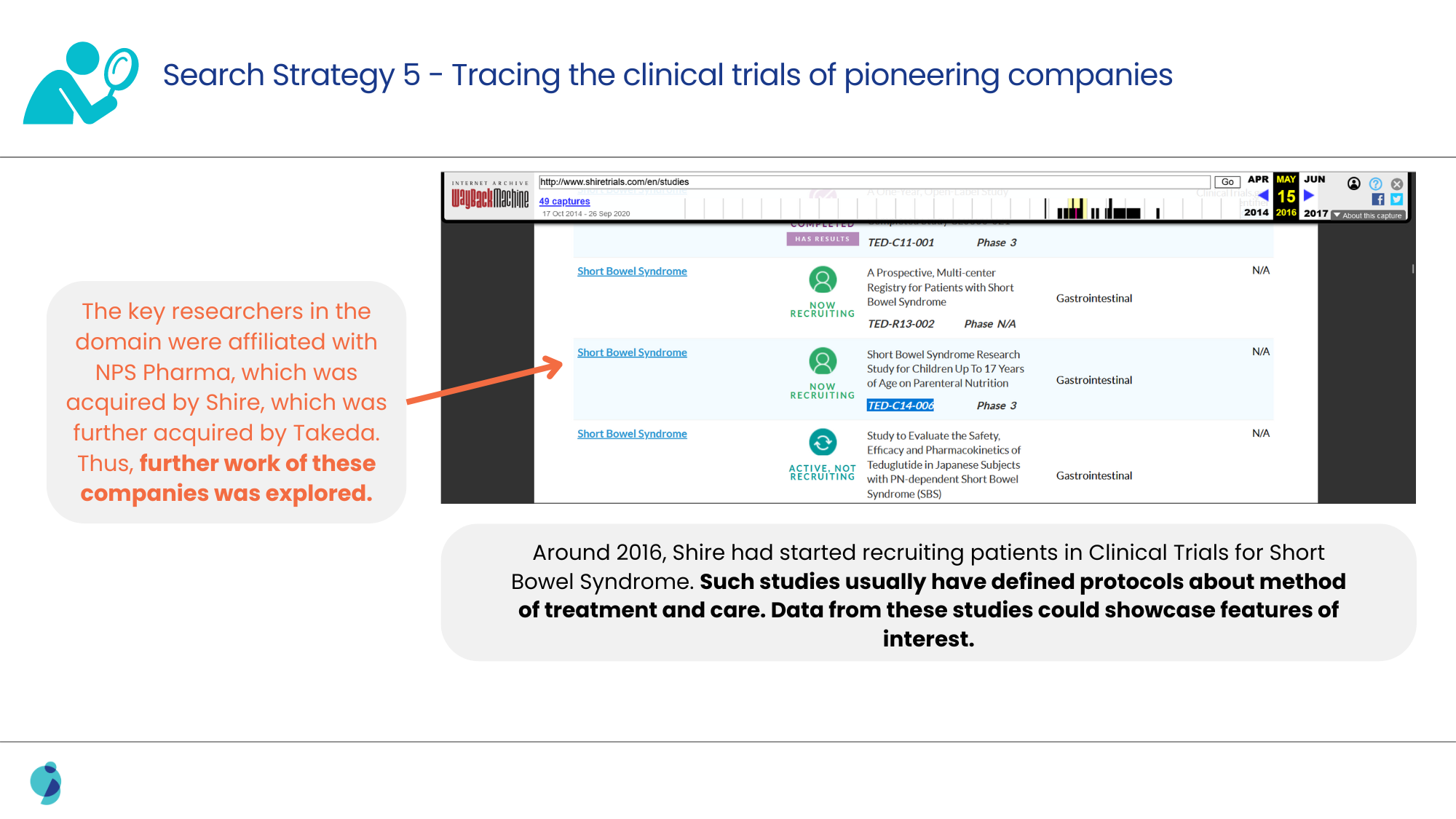
When the usual alternatives to conventional search strategies, like exploring posters, meeting abstracts, and conferences, failed, a deeper search for clinical trials was performed.
Analyzing the methodologies discussed in such clinical trials led us to confirm the link between the surrogate marker and the therapy, as well as the frequency of adjustment for the patients.
Get the full case study to discover how GreyB successfully invalidated a patent on the dosage regimen of a multimillion-dollar drug to help in generic drug entry.






