If Drug research and development is your bread and butter, you must be familiar with the wonders of Stelara.
For the uninitiated, Stelara is the brand name of the drug Ustekinumab, which is developed by Janssen Pharmaceuticals, a subsidiary of J&J. It is used for the treatment of Crohn’s disease, ulcerative colitis, plaque psoriasis, and psoriatic arthritis in 18+ year-old adults.
The drug was first approved in 2008 and is sold globally, including in the US, UK, France, Germany, and Japan.
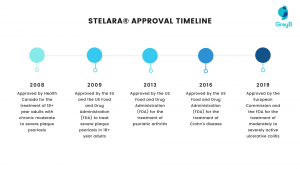
Here is the timeline for which it was approved by different countries for different diseases:
- On December 12, 2008, by Health Canada for the treatment of 18+ year adults with chronic moderate to severe plaque psoriasis.
- In 2009, the EU and the US Food and Drug Administration (FDA) also approved the drug to treat severe plaque psoriasis in 18+ year adults.
- On September 24, 2013, the US Food and Drug Administration (FDA) approved the drug for the treatment of psoriatic arthritis. In September 2016, the FDA approved it for treating Crohn’s disease.
- In 2019, the European Commission and the FDA approved it for the treatment of moderately to severely active ulcerative colitis. (Source)
Just how important is Stelara for the Company?
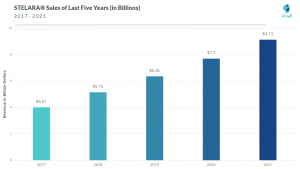
Interestingly, almost ⅔ of its sales come from the US. It is one of the few drugs with a revenue of more than a billion dollars for the last 5 years. Even in 2021, Stelara has sales of $9.13 billion, an 18% jump compared to its 2020 sales.
The reason for its multi-billion-dollar sales is that the drug has no generic version developed as the patents securing the drug are still in effect.
But not for long; the biologics patents expiration list shows that Stelara’s patents are starting to expire next year.
Like every drug company, Johnson & Johnson also filed multiple patents around the drug and made a patent thicket. Here’s how it looks:
| Indications | Psoriatic Arthritis | Ulcerative Colitis | Plaque Psoriasis | Crohn’s disease | Systemic Lupus Erythematosus (SLE) |
| Composition of Matter | US6902734B2
Expires Sep 25, 2023 |
||||
| Indication/ Method of Treatment | US11197913B2
Expires Mar 28, 2037 |
US10961307B2
Earliest Expiry: Sep 24, 2039 |
US20200331996A1
Under Application stage |
US8557745B2
Earliest Expiry: February 16, 2032 |
US20190092853A1
Under Application stage |
| Formulation | ECSP10010056A
Under Application stage |
||||
| Manufacturing | US11079361B2, US20200291107A1
Earliest Expiry: June 22, 2039 |
||||
| Other (Delivery Device, etc.) | 9 patents (EP3873572A1, EP3873573A1, EP3873570A1, EP3873571A1, US20210030967A1, WO2021059214A1, WO2021059212A1, WO2021059210A1, WO2021095003A1)
Expire 2024-2032 |
||||
Conclusion
Yes, the drug patent will soon run out of its days. The drug, however, will still hold immense value, and the best part is it’ll soon be up for grabs in the generic drug market. With the explosive growth in the generic drug industry, companies (like yours) are in a battle to claim a share of this soon-to-be-expired drug as quickly as possible.
Tracking patent expirations beforehand can not only help evaluate business opportunities but also help in refining market entry strategies. But there still lies legal as well as technology battles for your company to win before you can proceed with the generic development of Stelara.
With proper research and threat analysis, the nightmare of developing a generic version can turn into a fruit-bearing reality. How to go about it? Well, let’s start by getting in touch by filling out the form below, and we’ll get back to you in no time.