The global medical device industry will reportedly be worth $801.4 billion by 2031!
According to Statista, there are an estimated 2 million different kinds of medical devices on the world market. BMI research estimates that about 5,500 active companies operate in this sector, 4,465 of which are based in the United States.
Having so many players in the market, it gets tricky to identify new innovators with incredible potential and value. In this analysis, we identify some noteworthy medical device companies, exploring their remarkable strides in healthcare.
We will discuss their critical R&D areas, the transformative technologies they employ, and their tangible impact on patients, medical practitioners, and the industry.
Explore the latest innovation trends in the 2024 digital healthcare industry and the entities working on it. Fill out the form below to get our complete report emailed directly to you!
10 Startups Researching Medical Devices:
1. LegWorks
| Website | |
| HQ | New York, United States |
| Total Funding Amount | $2.7 Million |
| Top Investors | 43North, Grand Challenges Canada, Ontario Centres of Excellence, Holland Bloorview Kids Rehabilitation Hospital, Toronto Innovation Acceleration Partners, Grand Challenges Canada |
| Innovative Tech | Incorporates a patented stance-phase control system (AutoLock) and a swing-phase control mechanism (Variable Cadence Controller) |
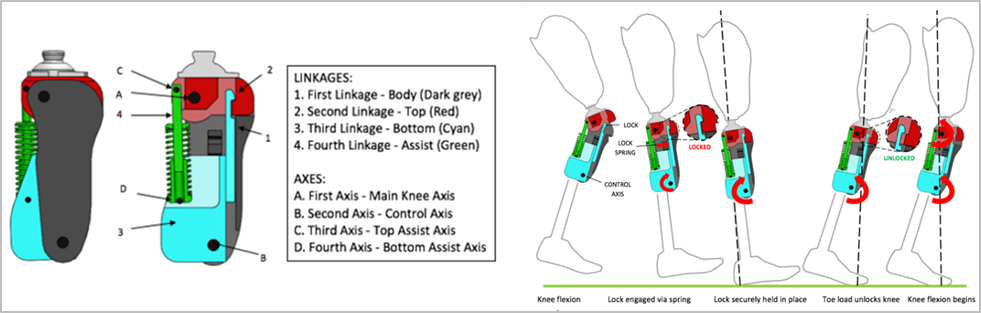
LegWork’s Innovative Technologies
LegWorks debuted its AutoLock Technology in its hydraulic knee joint product lines. The All-Terrain Knee incorporates a patented stance-phase control system (AutoLock) and a swing-phase control mechanism (Variable Cadence Controller or VCC). AutoLock technology operates on a knee lock that engages automatically in the late-swing phase for added safety.
The Variable Cadence Controller (VCC) combines a variable friction mechanism with an improved extension support spring to provide effective gait at various walking speeds.
The products offered by LegWorks include – All-Terrain Knee, All-Terrain Knee Premium, All-Terrain Knee Premium HD, ATK HydraPro, and ATK HydraPro HD.
The AutoLock Technology
What challenges do LegWork’s patents solve?
Artificial joints typically need mechanisms to control movement. For instance, someone with an above-knee or through-knee amputation may require an artificial knee or prosthetic joint.
These joints should allow for bending during activities like sitting, kneeling, and walking. Effective control during walking or running is also essential to prevent excessive heel lifting and impact.
Better control can lead to a more natural walking pattern. Additionally, the joint should offer stability and structural support. Therefore, there’s a demand for enhanced joint structures and controllers.
Legworks’ patent solves this problem with an artificial joints device. The device has a controller that can change how easy or hard it is for the joint to move.
For example, when someone walks, this device can make it feel more natural and stable. It can also help control how much effort is needed to move the joint. This can be useful for people with artificial legs or arms.
LegWork’s Collaborations
Institute for Global Orthopaedics and Traumatology
IGOT (Institute for Global Orthopaedics and Traumatology) has collaborated with Legworks to conduct a prospective study. This study evaluates the costs and benefits of providing prosthetic solutions for above-knee amputees.
The partnership anticipates that the data collected would effectively strengthen the case for advocating enhanced accessibility to prosthetics in underserved regions with limited resources, such as Tanzania (Source)
2. ComeBack Mobility
| Website | comebackmobility.com |
| HQ | New York, United States |
| Total Funding Amount | $1.1 Million |
| Top Investors | Dmytro Savienkov, Anton Zheliabin, Mark Magajne, Antipov Maxim, Sergey Berezhnoy, Myron Jarosewich, Peter Chernyshov, Olga Golikova, Rostyslav Maikovych, Anton Bartieniev |
| Innovative Tech | Smart Crutch Tips is an innovative tool for establishing and monitoring patient compliance |
Comeback Mobility’s Innovative Technologies
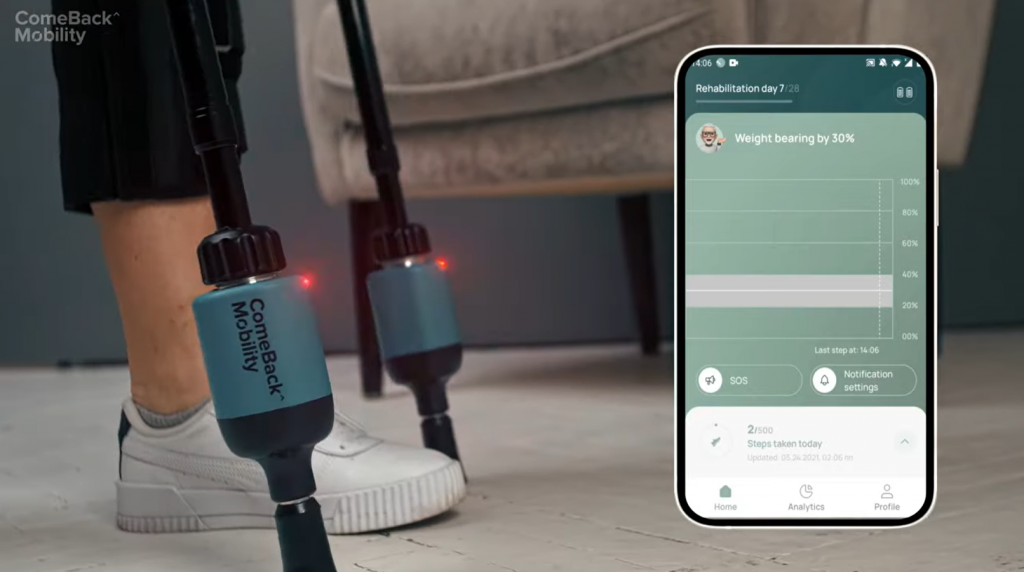
Credit: ComeBack Mobility demo video
ComeBack Mobility launched Smart Crutch Tip Devices to revolutionize the rehabilitation sector. The weight-bearing service provided by the company makes the healing process safe and clear for both doctor and patient.
It is a Medical Device Class II, FDA registered, and has all essential regulatory clearances in the United States.
Smart Crutch Tips is an innovative tool for establishing and monitoring patient compliance while achieving the required weight-bearing status.
The data recorded on the tips is delivered to the doctor’s phone and PC dashboard using Smart Crutch. It assists the doctor in developing a weight-bearing (WB) program for the patient’s recovery.
3. Proov
| Website | |
| HQ | Colorado, United States |
| Total Funding Amount | $10 Million |
| Top Investors | Lightship Capital, GingerBread Capital, SteelSky Ventures, WCC Partners, Hambrecht Ducera Growth Ventures, Kim Perell, National Science Foundation, SteelSky Ventures, Texas HALO Fund |
Proov’s Innovative Technologies
The Proov Complete Testing System is their most in-depth at-home fertility test. It offers complete cycle hormone insights for all four main cycle hormones and hormone markers: FSH (follicle stimulating hormone), E1G (estrogen marker), LH (luteinizing hormone), and PdG (progesterone marker).
Proov Complete employs a patented testing procedure to certify successful ovulation by PdG testing. This testing kit identifies the initial PdG spike after an LH surge to confirm ovulation and tracks PdG levels throughout the implantation window. The data collected is uploaded to the Proov Insight app. (Source)
What challenges does Proov’s patents solve?
Only 25% of women needing fertility treatment can get it. Checking hormones remotely is essential for helping people with fertility issues.
Previously, women had to go to special labs to check their hormone levels, which often meant traveling long distances and missing work. Another option was mailing blood samples to a lab, which takes too long. Effective fertility treatment needs quick results. These solutions for fertility issues are very time-consuming.
One of Proov’s patents discusses a system for tracking ovulation at home. A person can also check if they are pregnant without medical knowledge. The system uses a smart device to analyze different substances in a sample on a test strip.
Another problem one of its patents discusses is measuring progesterone by detecting PdG.
Hormones like FSH, LH, estradiol, and progesterone control the menstrual cycle. These hormones can be measured in urine to track the cycle. FSH starts it, LH triggers ovulation, and progesterone prepares the uterus for pregnancy. After implantation, the hCG hormone appears.
People can use urine tests to track these hormones at home. Still, there’s a need for a test that measures explicitly progesterone by detecting PdG.
Proov’s patent aims to create a home urine test strip (PdG test strip) that helps women simply monitor their progesterone levels. It uses antibodies and hormones on a unique strip. You put a urine sample on the strip, which moves through the strip, showing results through colors. After using it once, you can throw it away.
This test strip can track progesterone levels during the menstrual cycle, helping identify fertile and non-fertile phases. It can also show if someone is pregnant.
Additionally, it helps detect menopause or issues with ovulation. In certain cancers, it can even detect progesterone. The test strip is designed to give clear, visible results.
Proov’s Collaborations
Quest Diagnostics
In July 2023, Proov partnered with Quest Diagnostics’ consumer-initiated testing division to introduce the Proov Confirm PdG home collection kit for fertility assessment via questhealth.com.
This pioneering collaboration marked the debut of a third-party women’s health test on the Quest platform, showcasing the Proov Confirm PdG test as the first FDA-cleared progesterone metabolite (PdG) home test kit designed to validate successful ovulation. It emerged as a response to the growing needs of women seeking to conceive or expand their families, aiming to offer a reliable and accessible solution through Quest’s platform, which predominantly features Quest Diagnostics’ laboratory-based test services.
I created Proov after my own battle with infertility because I wanted other women to have more information and options than I had at the time. We are thrilled to collaborate with Quest to broaden access to Proov Confirm so even more women can access insights to help them realize their dream of raising a family.
– Amy Beckley, Proov’s Founder, and CEO.
Awards and Recognition
Proov has won several awards, including “Best Mom Invention” at CES 2019 and the 2019 Startup Night SXSW pitch slam. They were also finalists in the Fourth Annual Best of Baby Tech Awards at CES 2019 and received the Parents Best Family Tech Award at CES 2019.
4. Senzo
| Website | |
| HQ | California, United States |
| Total Funding Amount | $3.9 Million |
| Top Investors | BioAdvance, Wellness Coaches USA, Bossanova Investimentos, Luigi Bajetti, Jay Zaveri, SOSV, IndieBio |
| Innovative Tech | Offers an innovative lateral flow test and technology similar to lab-based PCR test equipment |
Senzo’s Innovative Technologies
Senzo invented a groundbreaking lateral flow test and technology with the same accuracy as laboratory-based PCR. The Senzo Amplified Lateral Flow (ALF) equipment offers a unique approach for binding, detecting reagents, and capturing antibodies in the test zone. Furthermore, the ALF platform provides the most precise lateral flow rapid test. The Senzo Amplified Lateral Flow test is highly accurate in terms of PCR.
Even the Senzo Mobile Analyzer Platform (MAP) utilizes unique microfluidic test cartridge technology to provide highly reliable protein biomarker assays on-site with only a nasal swab, saliva, or small blood sample. The Senzo Microfluidic Cartridge has a patented 8-layer structure that provides unrivaled sensitivity. (Source)
Senzo Amplified Lateral Flow (ALF) + Senzo Mobile Analyzer Platform (MAP)
What challenges does Senzo’s patent solve?
Senzo’s patent solves one of the challenges related to microfluidic devices and valves that control fluid flow in small spaces. It’s used for detecting specific factors in fluids, like blood, on a very small scale. This is super useful for medical tests because it requires less fluid and fewer chemicals compared to larger-scale tests.
For example, finger puncture blood droplets can be used instead of single syringe blood for many medical examinations. These small devices can be used at a doctor’s office or home for quick tests.
Senzo’s Collaborations
BARDA
In 2022, Senzo announced a significant partnership with BARDA, an Administration for Strategic Preparedness and Response (ASPR) division under the US Department of Health and Human Services (HHS).
The collaboration aimed to facilitate the path to achieving the US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for Senzo’s Amplified Lateral Flow (ALF) COVID-19 test.
With support from federal funds provided by the Department of Health and Human Services and BARDA, this partnership underscored their shared commitment to advancing rapid and accurate diagnostic solutions and contributed to public health.
Abingdon Health PLC
In March 2023, Senzo Health Limited and Abingdon Health PLC entered a strategic partnership agreement. The collaboration entailed Abingdon Health extending its contract development and manufacturing (CDMO) services to Senzo and its partners.
This partnership facilitated the development and production of new rapid tests while leveraging Senzo’s advanced ALF platform. By combining Senzo’s innovative technology with Abingdon Health’s expertise in CDMO, the collaboration aims to drive the creation of advanced diagnostic solutions, enhancing the healthcare landscape with efficient and accurate testing methods. (Source)
5. EvoEndo
| Website | |
| HQ | Colorado, United States |
| Total Funding Amount | $10.6 Million |
| Top Investors | GI Opportunity Fund 1, MedTech Innovator |
| Innovative Tech | Offers a Gastroscope and Controller endoscopic diagnosis, therapy, and video monitoring |
EvoEndo’s Innovative Technologies
The EvoEndo® Model LE Gastroscope is designed to visualize the upper digestive tract in adults and children, primarily for the observation, diagnosis, and endoscopic treatment of the esophagus, stomach, and duodenum. Additionally, the EvoEndo® Controller is designed to work with an EvoEndo® Endoscope for endoscopic diagnosis, therapy, and video monitoring.
The EvoEndo Scope is a sterile, single-use technology that does not require repairs or reprocessing. Its Gastroscope’s operating channel can accept most standard pediatric biopsy forceps. With its four-way deflection abilities, this device allows doctors to conduct an unsedated examination of the esophagus, stomach, and small intestine.
The technology is designed to be user-friendly, eliminating the need for repairs or reprocessing and streamlining the medical equipment process. (Source)
EvoEndo® Model LE Gastroscope + Endoscope

EvoEndo’s Collaborations
Micro-Tech Endoscopy USA, Inc.
In 2021, EvoEndo entered into a significant partnership with Micro-Tech Endoscopy USA, Inc. The collaboration involved the distribution of EvoEndo’s innovative Endoscopy System by Micro-Tech in the US. This strategic partnership between the two companies aimed to revolutionize endoscopic procedures.
The innovative approach combined a sterile, single-use, flexible, ultra-slim gastroscope with virtual reality patient entertainment. This innovation enabled safe and anesthesia-free procedures for both children and adults.
Routine upper endoscopic procedures, many of which take minutes to perform, today almost always involve anesthesia for pediatric patients or conscious sedation for adults, which places immense burdens on patients, hospitals, and facilities alike. We look forward to reducing these burdens by eliminating the complexities and high costs associated with endoscopies and leveraging Micro-Tech’s US distribution network to significantly expedite physician and patient access to our system once we receive clearance from the FDA.
– Heather Underwood, Chief Executive Officer at EvoEndo
Awards and Recognition
EvoEndo has received notable recognition for its contributions to medical technology. In 2022, the company was honored with the MedTech Innovator Execution Award and the Virginia Shimer Rybski Memorial Award. Both prestigious awards acknowledge EvoEndo’s potential as an emerging entrepreneur in the field of medical technology and its dedication to enhancing patient care. (Source)
Companies like these are often on the acquisition radar of mega-corporations like Apple.
Discover how the Cupertino tech giant plans to dominate medtech in the foreseeable future.
6. ABLE Human Motion
| Website | |
| HQ | Catalonia, Spain |
| Total Funding Amount | $187.54 Million (€2.1 Million) |
| Top Investors | Horizon 2020, CaixaImpulse, Ministerio de Economia y Competitividad, Centre for the Development of Industrial Technology (CDTI), EIT Health, GENESIS Ventures, BStartup, EASME – EU Executive Agency for SMEs |
| Innovative Tech | Offers exoskeleton technology to equip persons with impairments to assist in their movements |
ABLE Human Motion’s Innovative Technologies
ABLE Human Motion designed and produced a patented exoskeleton technology to equip persons with impairments by allowing them to move more freely and independently.
The firm’s ABLE Exoskeleton is one of the world’s first lightweight and affordable robotic exoskeletons for clinical rehabilitation, allowing persons with mobility problems to walk again. It comes with ABLE Care, a cloud-based mobile software that provides personalized and data-driven therapy.
Furthermore, this technology has been verified at top European clinical institutes, demonstrating enhanced clinical outcomes and rehabilitation advantages. The mobile app assesses real-time gait performance and collects and monitors patient data. (Source)
ABLE Exoskeleton

What challenges does ABLE Patent solve?
Spinal cord injuries affect millions of people globally, often robbing them of the ability to walk and participate in their communities.
Robotic exoskeletons are emerging as promising solutions. These wearable devices help individuals with spinal cord injuries practice walking. They rely on sensors to measure balance and movement, assisting users in their steps.
While some exoskeletons are available in hospitals, others are in development or awaiting certification for broader use. Challenges include their size, weight, and the need for users to initiate steps with weight shifts, affecting balance and usability.
Able’s patent discusses an exoskeleton for patients. It has parts for the shanks, thighs, knees, hips, and foot soles. There’s also a controller that uses sensors to control the knee joints. When the system senses a user trying to take a step forward, it helps them walk naturally.
ABLE Human Motion’s Collaborations
Toyota Accelerator Program
In 2021, ABLE Human Motion joined the Toyota Accelerator Program after being selected for the Toyota Startup Awards. Despite the program’s completion, their collaboration with Toyota persisted, involving testing their ABLE Exoskeleton with Toyota-sponsored Paralympic athletes to gather feedback for improvement.
This partnership aimed to develop a tailored product meeting athletes’ needs. Additionally, ABLE Human Motion explored collaborations with the Paris 2024 Paralympic Games and the International Paralympic Committee, showcasing their dedication to advancing exoskeleton technology within the Paralympic arena. (Source)
RYSEN
In 2022, ABLE Human Motion engaged in a collaborative testing initiative with RYSEN, resulting in a pioneering partnership to enhance gait training experiences for individuals with spinal cord injuries. This collaboration materialized through a testing session at the European Centre of Neurosciences, uniting clinicians and engineers.
By combining the innovative technologies of the RYSEN and the ABLE Exoskeleton, the collaboration aimed to create an effective and safe training environment that is focused on restoring natural gait in individuals with spinal cord injuries. (Source)
Heidelberg University Hospital and Sint Maartenskliniek
In 2023, ABLE Human Motion partnered with Heidelberg University Hospital and Sint Maartenskliniek (SMK) for active involvement in the 62nd International Spinal Cord Society Annual Scientific Meeting (ISCoS 2023).
Working together, they served as speakers and exhibitors, aiming to exchange expertise. Its contributions ranged from Franziska Herzog’s poster on lower-limb exoskeleton use in daily scenarios to a workshop led by Joan Lobo. This workshop focused on exoskeleton applications for individuals with spinal cord injuries, featuring insights from SMK researchers on vibrotactile feedback and clinical improvements of exoskeletons. (Source)
Awards and Recognition
ABLE Human Motion has earned multiple notable awards, including Best European Robotics Startup at ERF2020, Special Mentions in Toyota Startup Awards, and Most Promising Solution at Brain Health for Life.
Additionally, they have been recognized as Catalonia’s Best Healthcare Startup, secured the NEOTEC 2019 grant from CDTI, and achieved 2nd Prize in the health and biotechnology category at the Expansion Startup Awards. They have been featured among the top Barcelona startups to watch in 2020 and highlighted as one of seven rising exoskeleton startups in Europe. (Source)
7. Garwood Medical Devices
| Website | |
| HQ | New York, United States |
| Total Funding Amount | $14.3 Million |
| Top Investors | MedTech Innovator, The Murray Family, WNY Impact Investment Fund |
| Innovative Tech | Offers BioPrax to investigate the removal of biofilm infections on prosthetic knee implants and help retain the original implant |
Garwood’s Innovative Technologies
The company helps patients suffering from bacterial biofilm infections by utilizing innovative technology that allows treatment without removing their implants. These technologies are currently under investigation by FDA for marketing approval. Garwood Medical Devices launched BioPrax™ to investigate the removal of biofilm infections on prosthetic knee implants during early intervention treatments while preserving the current standard of care.
What challenges does Garwood’s patent solve?
Quickly healing wounds is essential to enhance patient comfort, reduce the risk of reopening or reinjury, and minimizing scarring. However, wound healing can be challenging as some wounds resist treatment. In addition, the existing electronic wound healing techniques involve multiple pieces of equipment, keeping patients tethered to cords. They also require separate bandages for protection and fluid capture.
These methods are underused because they are time-consuming to set up and often uncomfortable for patients. Garwood has a solution in the form of an improved, user-friendly wound treatment device that allows patient mobility and simplifies treatment.
This medical company’s patent discusses a wound healing system featuring a bandage designed for wound care and treatment. The dressing includes a layer with an absorbent pad for bodily fluids and incorporates at least two snap-button electrodes. These electrodes deliver a current across the wound to expedite the healing process. This innovative bandage enhances patient comfort, provides greater patient mobility, and simplifies treatment administration by trained healthcare professionals.
Its other patent aims to treat metal implants by passing electrical current through them to remove biofilms from their surfaces. However, a challenge arises when the implant is already inside a patient’s body, making it hard to access. The wires are inserted through the skin to contact the implant, but attaching anything to it may alter its function. Another challenge is ensuring the wire is touching the implant.
The patent introduces a capacitance-based method and device for detecting when a metal implant contacts a conductive wire or main needle while moving inside the body. As the main needle moves towards the implant, the capacitance gradually increases due to the body’s capacitance. However, when the main needle touches the metal implant, there is a sudden, noticeable spike in capacitance because the capacitance area now includes both the main needle and the implant. This capacitance spike is a clear indicator of direct contact with the implant.
BioPrax

Garwood’s Collaborations
Integrum AB
In 2021, Garwood Medical Devices joined forces with Integrum AB to explore a collaborative effort focused on expanding the treatment applications for the OPRA Implant System.
Garwood is known for its BioPrax technology targeting antibiotic-resistant bacterial biofilm infections associated with implants. And Integrum is skilled in improving amputees’ lives through the OPRA Implant System. This merger was to improve implant-related complications management and significantly enhance patient outcomes.
We are excited to announce this alliance with Integrum, which offers obvious benefits for both companies and, most importantly, for patients. We will benefit tremendously from Dr. Brånemark’s long experience as an orthopedic surgeon, researcher, and inventor. We will also collaborate to research another valuable application for our BioPrax™ implant biofilm infection control technology.
– Wayne Bacon, Co-Founder of Garwood Medical Devices Company
Buffalo Institute of Genomics (BIG)
In 2021, Garwood Medical Devices partnered strategically with Buffalo Institute for Genomics (BIG) to advance infection-related clinical outcomes. This partnership capitalized on Garwood’s innovative approach to disrupting the wound care and perio-prosthetic market.
Through this collaboration, BIG contributed state-of-the-art research and development capabilities and an accomplished university engineering team. This public-private partnership facilitated both Garwood’s and the University at Buffalo’s technologies through comprehensive research, design, and prototyping, focusing on validating and verifying applications and technologies. (Source)
Intel Corp. & Rosewell Park Cancer Institute
In 2017, Garwood Medical Devices collaborated with the University at Buffalo, Intel Corp., and Roswell Park Cancer Institute to develop an implantable sensor for detecting lung cancer.
Supported by a grant of $1 million from the National Science Foundation, the interdisciplinary team aimed to enhance wearable devices’ capabilities by combining implantable sensors, wearables, and software to identify severe illnesses like lung cancer more accurately. (Source)
Awards and Recognition
Garwood demonstrated its commitment to aiding prosthetic joint infection patients through BioPrax™ technology. In 2019, BioPrax received FDA Breakthrough Device Designation.
In 2020, they secured a $749,000 grant for their BioPrax device to combat bacterial biofilm infections linked to orthopedic implants.
Further, in 2022, they earned the Innovations for Vets Quickfire Challenge award from Johnson & Johnson Innovation – JLABS and the Johnson & Johnson Office of Military and Veterans Affairs.
In 2023, Garwood Medical Devices, LLC won the Buffalo Inno’s second annual Fire Awards.
Read more: Top medical device companies based on patents
8. Parasym Health
| Website | |
| HQ |
England, United Kingdom |
| Total Funding Amount | $94.8 Million |
| Top Investors | Undisclosed Investors |
| Innovative Tech | Offers a neurotechnology that treats neuromodulation while increasing the quality of life |
Parasym’s Innovative Technologies
The firm invented and offered Parasym neurotechnology that treats neuromodulation while increasing the quality of life. Parasym™ uses bioelectrical impulses directed at organ neural networks by stimulating the patient’s vagus nerve. The Parasym™ has drastically improved outcomes in some of the world’s most serious diseases. Let’s clearly understand the issue and how Parasym solves it.
ParasymTM

What challenges does Parasym’s patent solve?
The vagus nerve regulates various vital bodily functions. A Vagus Nerve Stimulation (VNS) treatment can stimulate it with electrical pulses.
VNS has shown promise in treating conditions like epilepsy, depression, pain, and more. However, traditional VNS involves surgical implantation, limiting its accessibility. Recent advances have introduced non-invasive methods like transcutaneous vagus nerve stimulation (tVNS), which is more accessible and cost-effective.
Yet, there are challenges. tVNS devices must be comfortable and stay in place during use. Safety, effectiveness, and usability issues, such as skin burns or ineffective stimulation, still need to be addressed.
Parasym’s patent talks about a system that stimulates the vagus nerve in a person’s body. It has a clip designed to attach to the tragus (part of the ear) with two arms that naturally press together. The arms have electrodes near their ends, and a signal generator connects to these electrodes. The signal generator produces therapy signals that go through the electrodes to stimulate the vagus nerve in the tragus, adjusting its activity.
Parasym’s collaborations
University of Oklahoma
In 2022, Parasym and the University of Oklahoma partnered for groundbreaking neuromodulation research in heart failure treatment. The trial, employing the Parasym device, notably improved cardiac mechanics and quality of life while reducing inflammatory markers for a patient group with limited treatment options. (Source)
9. Reperio Health
| Website | reperiohealth.com |
| HQ | Oregon, United States |
| Total Funding Amount | $6 Million |
| Top Investors | Liquid 2 Ventures, Caduceus Capital Partners, Travis Rush, G Ventures, Rogue Venture Partners |
| Innovative Tech | Offers Bluetooth-integrated biometrics, at-home screening tool, and onsite screening alternatives |
Reperio’s Innovative Technologies
The proprietary kit by Reperio provides full-service and combined biometric service at-home and onsite screening alternatives. CardioChek, PTS’ unique lipid and glucose sensor, is included in the package and may deliver a lab-quality lipid profile in as little as 90 seconds.
When used at home, the kit’s Bluetooth-integrated blood pressure cuff is automatic and helps to minimize stress. It is paired with other biometric data, such as waist and height measurements, to provide essential insights into general health. The breakthrough technology that drives Reperio’s flagship biometric home-based screening kit has earned the company its first patent.
Bluetooth-Integrated At-Home Screening Tool

What challenges does Reperio’s patent solve?
Many people skip physical exams due to travel, scheduling, work, financial concerns, or because they “feel fine”. Healthcare systems also deprioritize them.
Delaying or skipping routine physical exams has significant consequences. It reduces the chances of early diagnosis and timely treatment, making it harder to maintain a comprehensive medical history for future healthcare decisions.
Adopting portable or at-home diagnostic testing equipment faces obstacles like complexity and high costs, limiting access. Understanding test results, confirming correct execution, and combining multiple test outcomes for a useful health assessment are challenging, thus limiting the practical use of these solutions.
Reperio’s patent relates to automated modular systems for physical health testing, including devices and methods. In simple terms, these systems consist of a central unit that connects to various health testing devices. These medical devices measure your physical health and send the data to the central unit or your mobile device, allowing you to have a convenient health checkup at home or elsewhere.
Reperio’s collaborations
US Wellness
In 2022, Reperio Health partnered with US Wellness to offer at-home health assessment screening tools. This collaboration provided US Wellness clients with a convenient way to measure critical health metrics such as BMI, cholesterol, blood pressure, and more from their homes, using Reperio’s FDA-cleared testing kit. This partnership aligns with both companies’ aim to promote proactive health management and wellness among individuals and employers.
Health Designs
In 2022, Reperio Health partnered with Health Designs to offer various employers at-home health assessment screening tools. This collaboration brought Reperio’s convenient biometric screening solution, including FDA-cleared medical devices, to Health Designs clients, allowing individuals to assess their risk for preventable chronic conditions from the comfort of their homes. (Source)
In 2023, Reperio Health partnered with Access Care Health to integrate biometric screenings into their services. This collaboration enhanced Access Care’s offerings with Reperio’s FDA-cleared device and mobile app for immediate results, providing streamlined and comprehensive health assessments at various healthcare facilities.
We are thrilled to expand our offerings to clients overseas. The need for convenient access to biometric screenings is greater than ever, both domestically and internationally, and we see immense potential in servicing organizations that value preventive care interventions and early detection to make the greatest impact on population health.
– Travis Rush, CEO and Co-Founder of Reperio
10. CMR Surgical
| Website | |
| HQ | Cambridgeshire, United Kingdom |
| Total Funding Amount | $974.7 Million |
| Top Investors | Tencent, Ally Bridge Group, LGT Group, Chimera Partners, Cambridge Innovation Capital, RPMI Railpen, PFM Health Sciences, GE Healthcare, Watrium AS, SoftBank Vision Fund |
| Innovative Tech | Offers a next-gen surgical robot, allowing surgeons and hospitals to work optimally on patients |
CMR Surgical’s Innovative Technologies
Versius, a next-generation surgical robot, was developed by CMR Surgical. It was designed considering the needs of patients, doctors, and surgical teams. Its small-scale architecture, enabled by proprietary V-Wrist technology, is ideal for almost any operating room.
The technology allows surgeons and hospitals to work optimally on patients while maximizing utilization. It enables customized robotic and laparoscopic treatments based on patient requirements. As a result, it reduces the cost of performing robotic MAS while providing high-quality patient care. Sources: (1)(2)
CMR Surgical has filed over 1,000 patents, of which 295 were granted. Most patents discuss the startup’s surgical robots and the challenges they solve.
Explore all of CMR Surgical’s active patents from Slate, our intuitive patents analysis tool.
Click here to access the dashboard.
Versius By CMR Surgical
CMR Surgical’s collaborations
IRCAD
In 2021, CMR Surgical partnered with IRCAD, a leading research and training center for minimally invasive surgery, to integrate the Versius Surgical Robotic System into surgical training. This collaboration aimed to provide hands-on experience to over 6,200 surgeons annually, enhancing their robotic minimal access surgery skills and advancing the adoption of advanced surgical techniques. (Source)
Johnson & Johnson
In 2022, CMR Surgical partnered with Johnson & Johnson MedTech’s Ethicon business to jointly market and sell the Versius surgical robotics systems in specific markets. This collaboration aimed to leverage the expertise of both companies’ commercial teams and targeted select hospitals in Italy, France, Germany, and Brazil.
By combining CMR Surgical’s advanced robotics technology with Ethicon’s established market presence, the partnership aimed to provide innovative surgical solutions to healthcare facilities and enhance the accessibility of state-of-the-art surgical robotics across these regions. (Source)
Institut Curie
In 2022, CMR Surgical introduced a notable partnership with Institut Curie, France’s renowned cancer research center, marking a significant step in surgical innovation. This collaboration integrated the Versius Surgical Robotic System into a two-year clinical study, explicitly focusing on gynecological cancer surgery and minimally invasive hysterectomies. (Source)
Awards and Recognition
CMR Surgical has earned several notable awards and recognitions. In 2021, its Versius surgical robot received the iF DESIGN AWARD for the Medicine/Health category. The company was also honored with a King’s Award for Enterprise for Innovation. In 2018, CMR Surgical received the “Venture Financing Deal of the Year” title at the Medtech Insight Awards.
Conclusion
These medical device startup companies can aid even established players in fortifying their operations and product portfolios in the health tech industry, thereby improving patient outcomes. However, thousands of new medical device makers arise yearly, complicating scouting valuable startup innovators.
In this report, our analysts conducted in-depth research into the medical startup companies, their device’s effectiveness, patented technologies, and funding history, among many others.
Let us handle this time-intensive task of finding the right startups and filtering them down to match your specific needs so that you can focus on refining your innovation ideas for 2024.
Click the button below to get in touch with one of our experts!
Authored By – Vipin, Market Research
Edited By – Hemanth Shenoy, Marketing