While numerous drugs for diabetes are available in the market, many come with undesirable side effects such as weight gain, hypoglycemia, and hypertension. However, a game-changing solution has been introduced in the form of Farxiga®/Forxiga®.
Farxiga®/Forxiga® is the trade name for Dapagliflozin, by AstraZeneca. It is a sodium-glucose cotransporter 2 (SGLT2) inhibitor, representing a novel class of antihyperglycemic agents with an insulin-independent mode of action.
This drug entered the market in 2012 for the first time with the approval of EMA (Europe) and TGA (Australia), followed by Korea and the US.
Here is the timeline for which different countries approved Farxiga®/Forxiga® for different diseases:
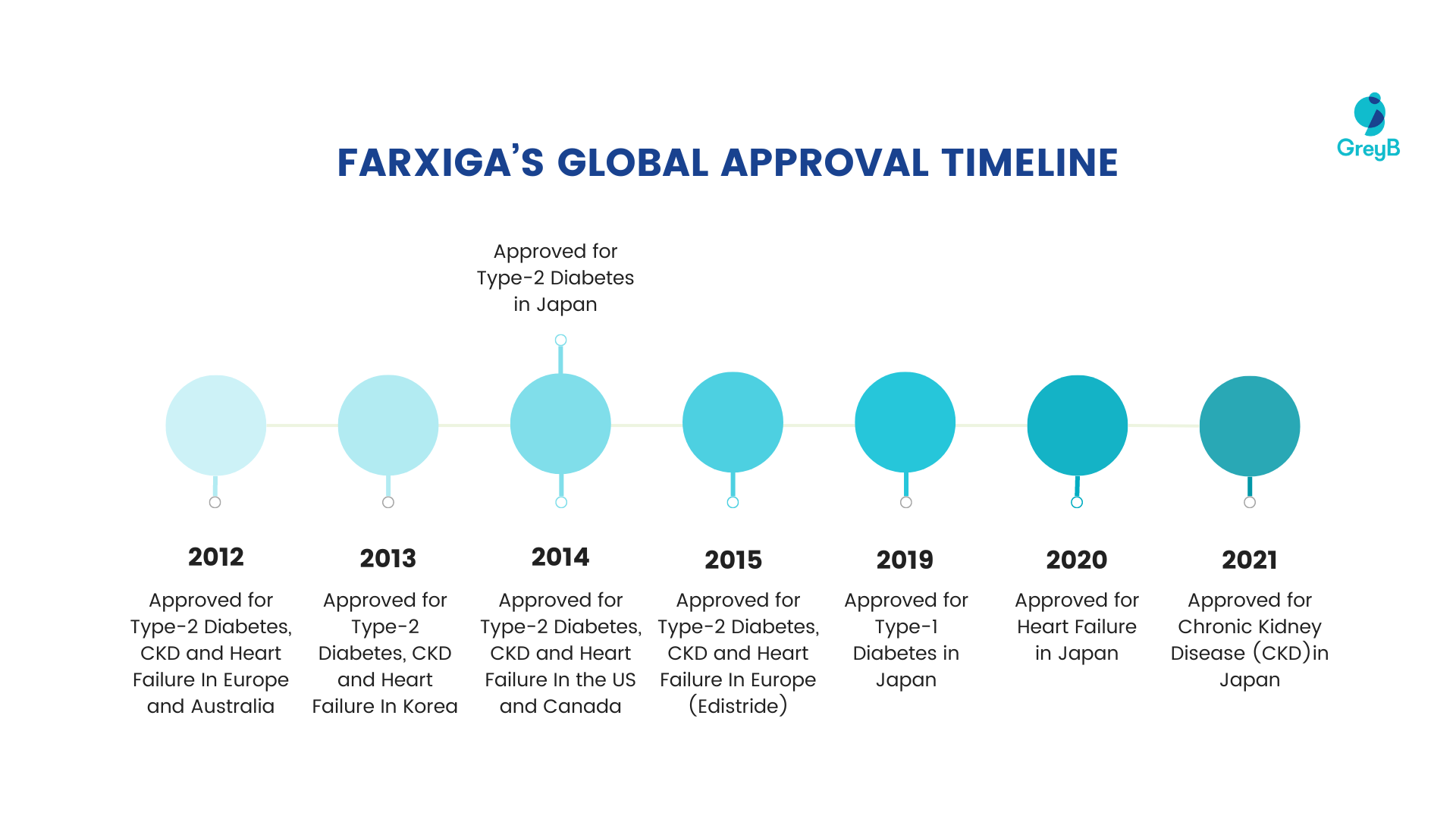
How important is Farxiga® to AstraZeneca?
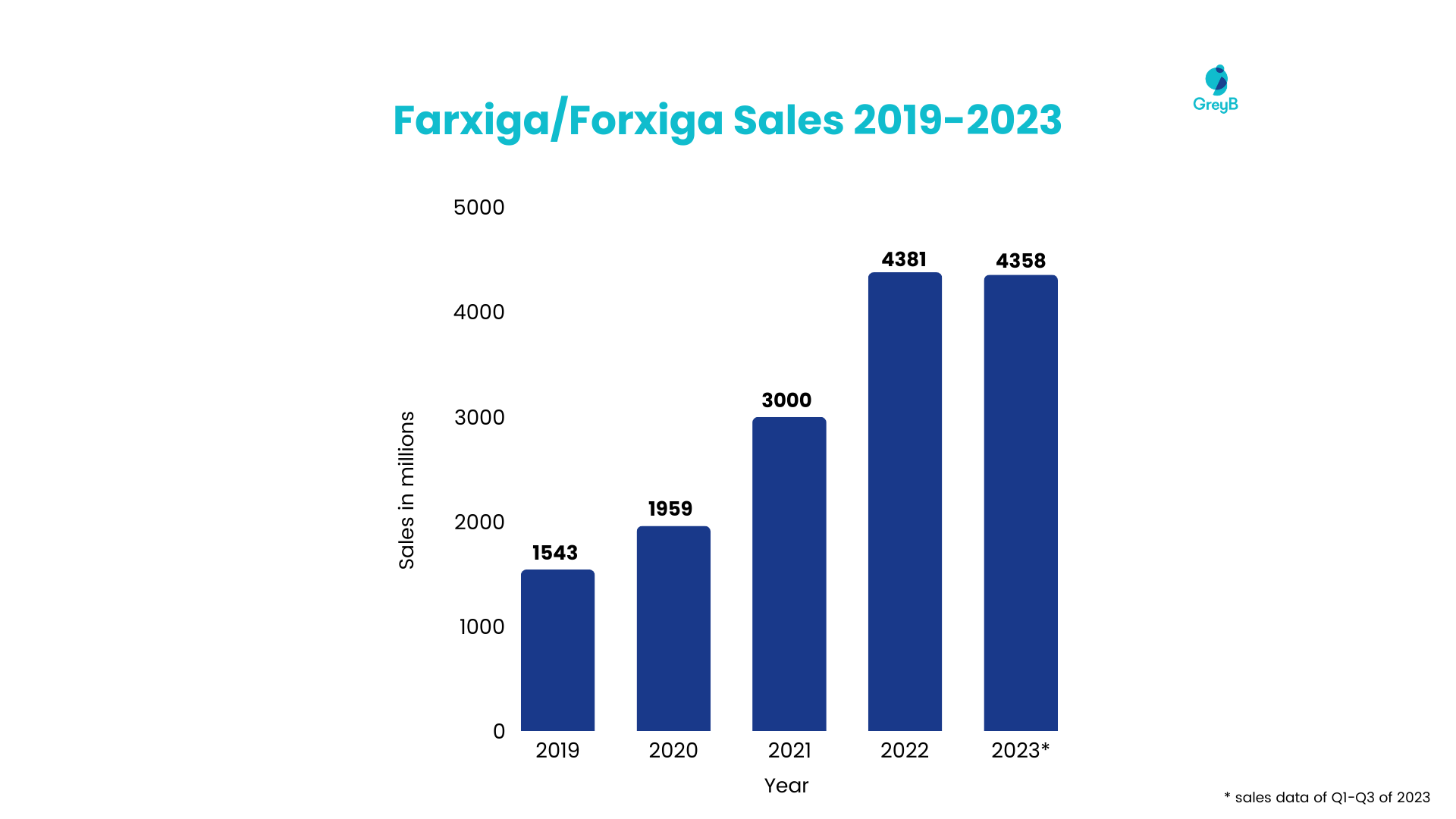
Farxiga®/Forxiga® is a leading product of AstraZeneca in diabetes management, chronic kidney disease, and heart failure and has witnessed a remarkable surge in sales (Fig. 1) over the past few years. The reason for its multi-million-dollar sales is its molecule patent.
Despite its dominance in the market, Farxiga®/Forxiga® faces challenges as its molecule patent approaches expiration next year, bringing opportunities for generic manufacturers to launch its generic version.
To protect its position and investment, like every drug company, AstraZeneca has strategically filed multiple patents around the drug, forming a patent thicket.
Here’s how it looks:
| Indications | Type 2 Diabetes | Chronic Kidney Disease | Heart Failure/Cardiovascular Disease | Other (Obesity, COVID 19, NASH) |
| Composition of Matter | US6515117B2 Expires on Oct 04, 2025 US7919598B2 (Crystalline form) Expires on Dec 16, 2029 | |||
| Indication/ Method of Treatment | US20170135981A1 Under Application stage | US8791077B2 Expires on Oct 04, 2029 US20210338648A1, US20230364077A1 Under Application stage | WO2023237512A1 Under Application stage | US8883743B2 Expires on Apr 04, 2026 US9757404B2 Expires on Sep 24, 2035 US20230165856A1 Under Application stage |
| Dosage Regimen | US11817195B2 Expires on Jun 13, 2039 US8685934B2 Expires on May 26, 2030 WO2023144722A1 Under Application stage | US20220023252A1 Under Application stage | US11826376B2 Expires on Jul 18, 2039 US10973836B2 Expires on Mar 09, 2040 | |
| Formulation | US8221786B2 Expires on Mar 21, 2028 || US9050258B2 Expires on Nov 12, 2030 || US8871264B2 Expires on Feb 23, 2031 || BR122017015103A2, RU2020102954A3 Under Application stage | |||
| Other | EP4315350A1 Under Application stage | |||
As top blockbuster drugs approach patent expiration, strategic ANDA filings are critical to secure early mover advantage. Download the Pharsight Digester report now for a deep dive into the patent strategies of the top 5 blockbuster drugs nearing expiration.
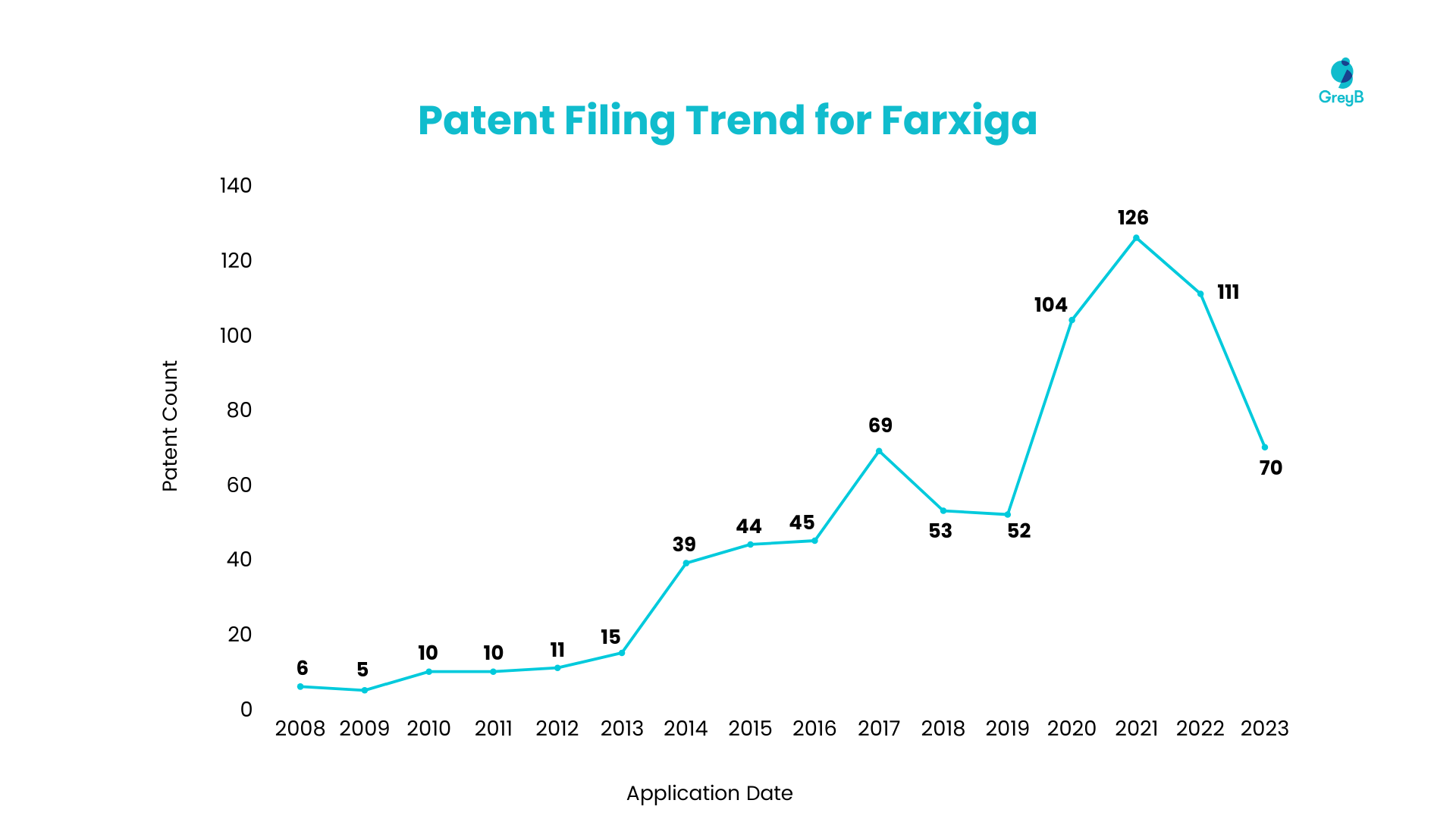
Patent Filing Trend for Dapagliflozin
Patent filing for Dapagliflozin started to increase in 2008, but the patent filing rate was steady. In later years, Dapagliflozin has seen growth in filing since 2013 and got a tremendous filing hike in 2022, resulting in 100+ patents being filed.
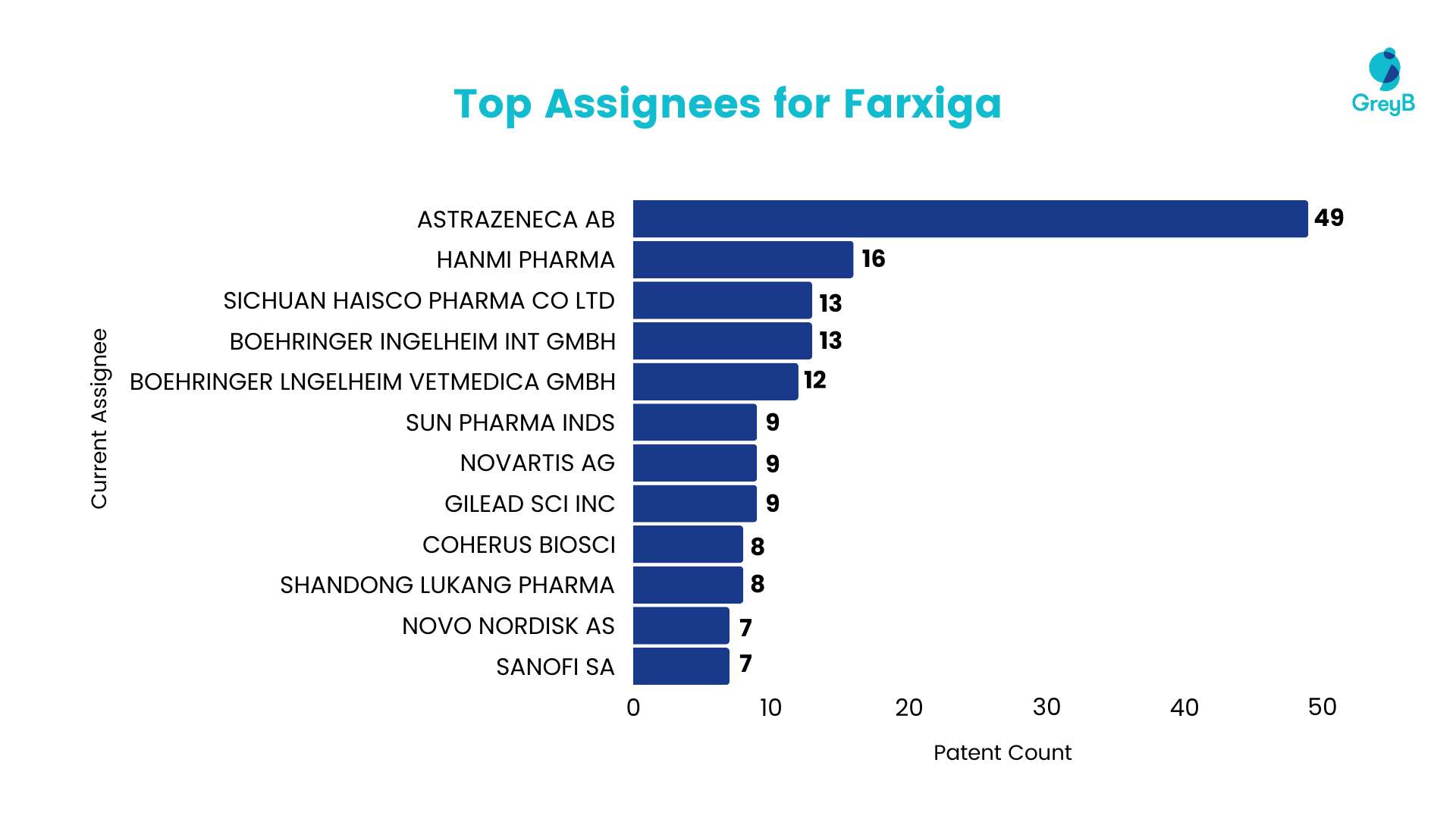
Top Assignees for Farxiga
AstraZeneca, the originating company actively filing patents for Dapagliflozin, holds over 40 patents related to the drug. Additionally, other notable companies engaged in patent filings for Dapagliflozin include Sichuan Haisco Pharma, Boehringer, Sun Pharma, Coherus Bioscience, Merck, and Sanofi, each with more than five patents. The participation of these major pharmaceutical companies in patenting Dapagliflozin underscores their keen interest in this medication.
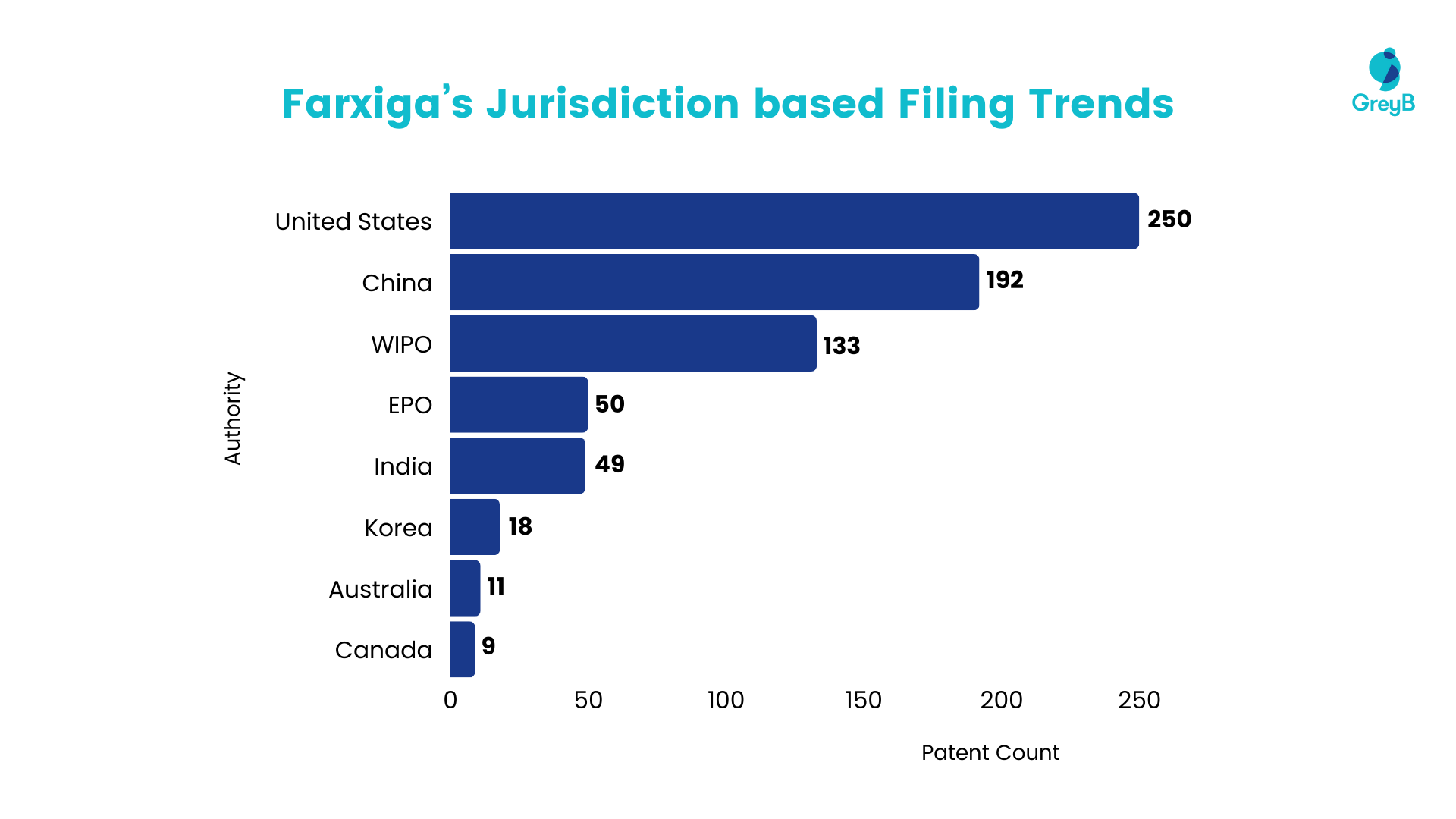
Filing Trend Based on Jurisdiction
Based on the data presented below, most patents have been filed in the United States and China, indicating a significant market focus for companies, with over 200 and 150 patents filed in each respective country.
Notably, India ranks fifth, with a patent count nearly equivalent to that of Europe, demonstrating considerable interest from companies in the Indian market for this drug. Given India’s lack of provision for patent term extension, this presents a substantial opportunity for generic manufacturers to swiftly enter the Indian market following the expiration of the molecule patent.
Clinical Trials
Dapagliflozin is an anti-diabetic drug, but it is also used for other indications like chronic kidney disease and heart failure. Interestingly, it is also being monitored for cancer and Fabry disease treatment. Both studies are in phase I of clinical trials. This suggests using Dapagliflozin for these diseases can be approved in the future.
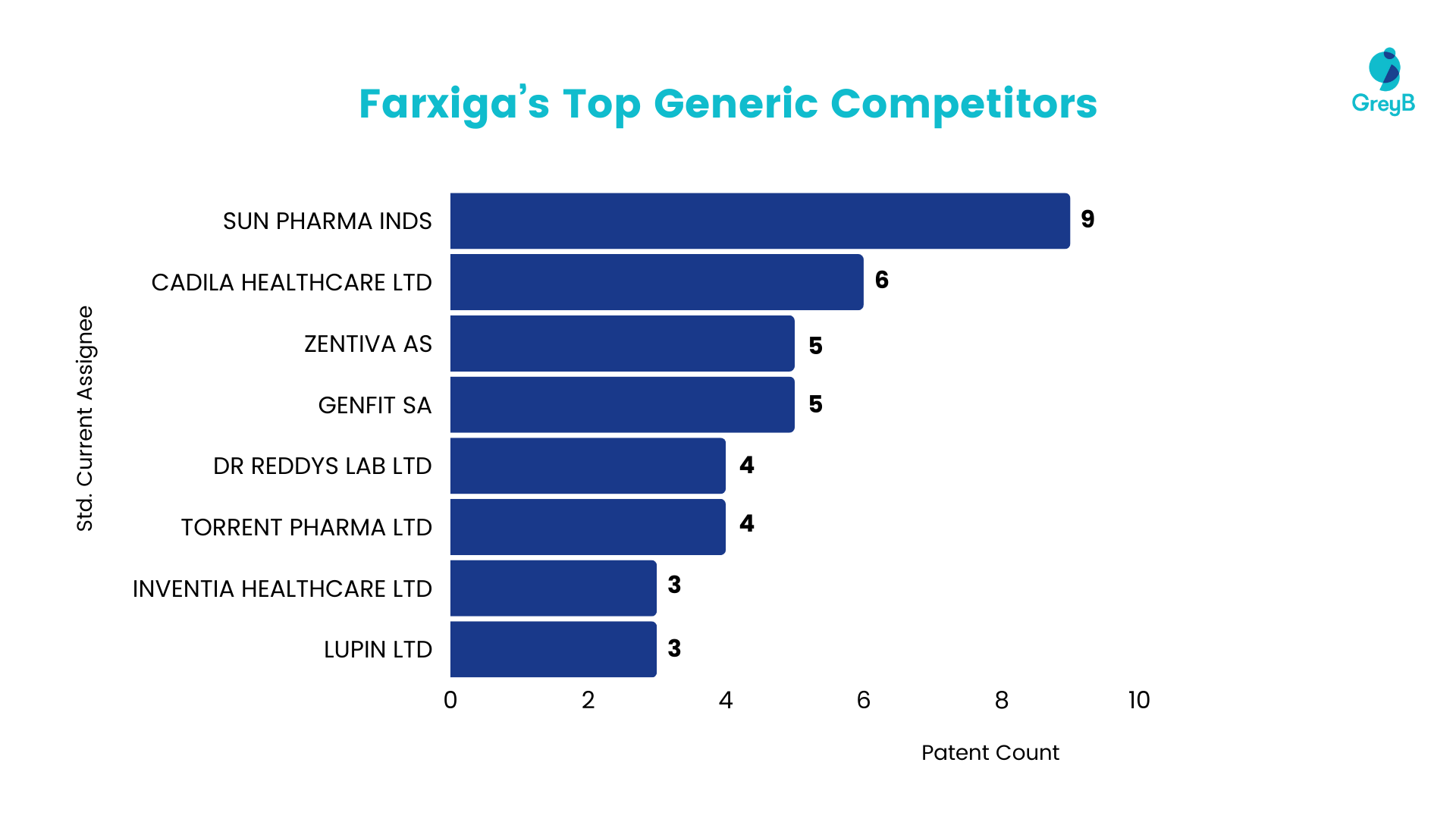
Generic Competitors
As seen in the data below, Sun Pharma is the most active participant in filing patents for this drug. Following that, other generic companies like Cadila, Zentiva, Genfit, and Dr. Reddy’s are also interested in this drug.
Interestingly, most of the companies are Indian generic companies that represent the Indian market as a significant focus for this drug.
Entry of Generic Version of Farxiga/Forxiga: A Prediction
The lapse of a crucial patent for Dapagliflozin means that generic manufacturers can now introduce their versions, offering more affordable options for consumers. It’s worth noting that Dapagliflozin’s formulation patents, both for monotherapy and combinations, remain valid until March 2028 and November 2030, respectively.
While companies can submit generic versions for approval by regulatory agencies, they can only be launched once the formulation patents expire. In the meantime, attention should be paid to any ongoing activities related to active patents.
Therefore, tracking patent expiration beforehand can not only help evaluate business opportunities but also help in refining market strategies. With this, generic manufacturers can position themselves to enter the market swiftly once all barriers are lifted.
That’s where our tool, Elixir, can help!
Elixir helps you stay vigilant and monitor activities related to the patents, giving you a headstart in the generic market.
With proper research and careful threat analysis, the extensive task of creating generic versions can transform into a fruitful opportunity.
Get in touch to see Elixir in action
Authored By – Shadab Riyaz, Life Sciences
Edited By – Ridhima Mahajan, Marketing
Also Read – The Trajenta Patent Thicket: Patents expiring in 2025










