A 2022 research review revealed that while the risk of contamination is relatively low for multi-use eye drop bottles within the first week of use (3%), the contamination rate increases significantly (24%) for bottles used over longer periods. This highlights the potential hazards associated with traditional preservative-reliant dispensers.
As a promising alternative, the packaging industry has explored the adoption of preservative-free technology, such as the MDPF (Multi-Dose Preservative-Free) dispenser. However, even with this innovative approach, contamination risks persist, particularly due to the presence of residual liquid droplets after dispensing, which could lead to contamination within the bottle.
To address these concerns, the packaging industry is actively working to mitigate the potential for contamination in MDPF systems, specifically addressing the issue of “Residual droplet” contamination.
Recent decontaminating innovations in eye drop dispensers
Aptar Pharma

Aptar Pharma has developed proprietary MDPF technology under the name “Ophthalmic Squeeze Dispenser,” which leading pharmaceutical companies trust for its ophthalmic products. These companies include Allergan, Bausch & Lomb, Optox, and Bayer.
To address “Residual Droplet” concerns, Aptar proposes two solutions:
- A dispenser featuring a removable ventilated cap that facilitates quick drying of residual droplets. The cap incorporates a sterile filter to prevent germ ingress and includes an integrated pad to absorb any leftover liquid at the dispensing opening (EP3730220B1).
- An anti-bacterial insert on the droplet-forming surface (US9833356B2).
Nemera; La Verpillière

Nemera, based in La Verpillière and a leader in MDPF technology, is known for its “Novelia® bottle” used by Alcon, Scope, Oasys Medical, and Optox for ophthalmic preparations.
To combat contamination from residual droplets, Nemera’s innovations include:
- Incorporating a reflux valve in the dispenser, allowing residual liquid to return to the reservoir from the dispensing valve (FR3103793A1).
- Applying an antimicrobial silver-ion-based surface coating on the dispensing tip (US9579671B2).
- Introducing a removable cap with an expulsion shape and absorption pad. This design directs residual liquid from the dispensing tip towards the absorption pad, which then evaporates through the expulsion shape (US9592934B2).
Silgan Dispensing

Silgan Dispensing, known for its Iridya™ MDPF dispenser, has introduced an innovative design element for the dispensing tip orifice, resulting in a smaller residual drop when the drop detaches (WO2023148055A1). Additionally, Silgan has proposed a vented cap design to expedite the evaporation of residual droplets on the dispensing tip, minimizing contamination (WO2023089479A1).
Kedalion Therapeutics
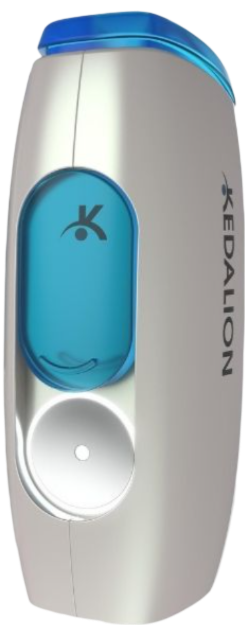
Kedalion Therapeutics developed the user-friendly AcuStream™ delivery system, known for its exclusive aiming mechanism that enhances dose precision and accuracy. The company introduced (US20220192874A1) a swivel cover with a flexible, antimicrobial-coated surface that minimizes contamination from residual droplets.
Future Outlook
Efforts are underway to develop effective strategies and solutions that can minimize the risk of contamination, ensuring the safe and hygienic use of ophthalmic solutions. The eye care industry is well-positioned to overcome these challenges through collaborative research, technological advancements, and a commitment to excellence.
Seeking reliable packaging partners for contamination-free ophthalmic products?
Let our experts guide you towards the ideal packaging solutions.
Authored By – Vikrant Saluja, Life Sciences
Edited By – Ridhima Mahajan, Marketing
Also Read – Tap Into The Loteprednol Etabonate Generic Drug’s Transformational Potential