Trajenta, known for its effective glycemic control without weight gain, is a key player in the diabetes medication sector. This critical medication, known generically as Linagliptin, is on the brink of its patent expiration, presenting an opportunity for generic drug manufacturers to introduce their own generic versions.
Manufactured by Boehringer Ingelheim, Trajenta® is an oral dipeptidyl peptidase-4 (DPP-4) inhibitor used in treating type 2 diabetes. Linagliptin can be used together with proper diet and exercise to treat hyperglycemia.
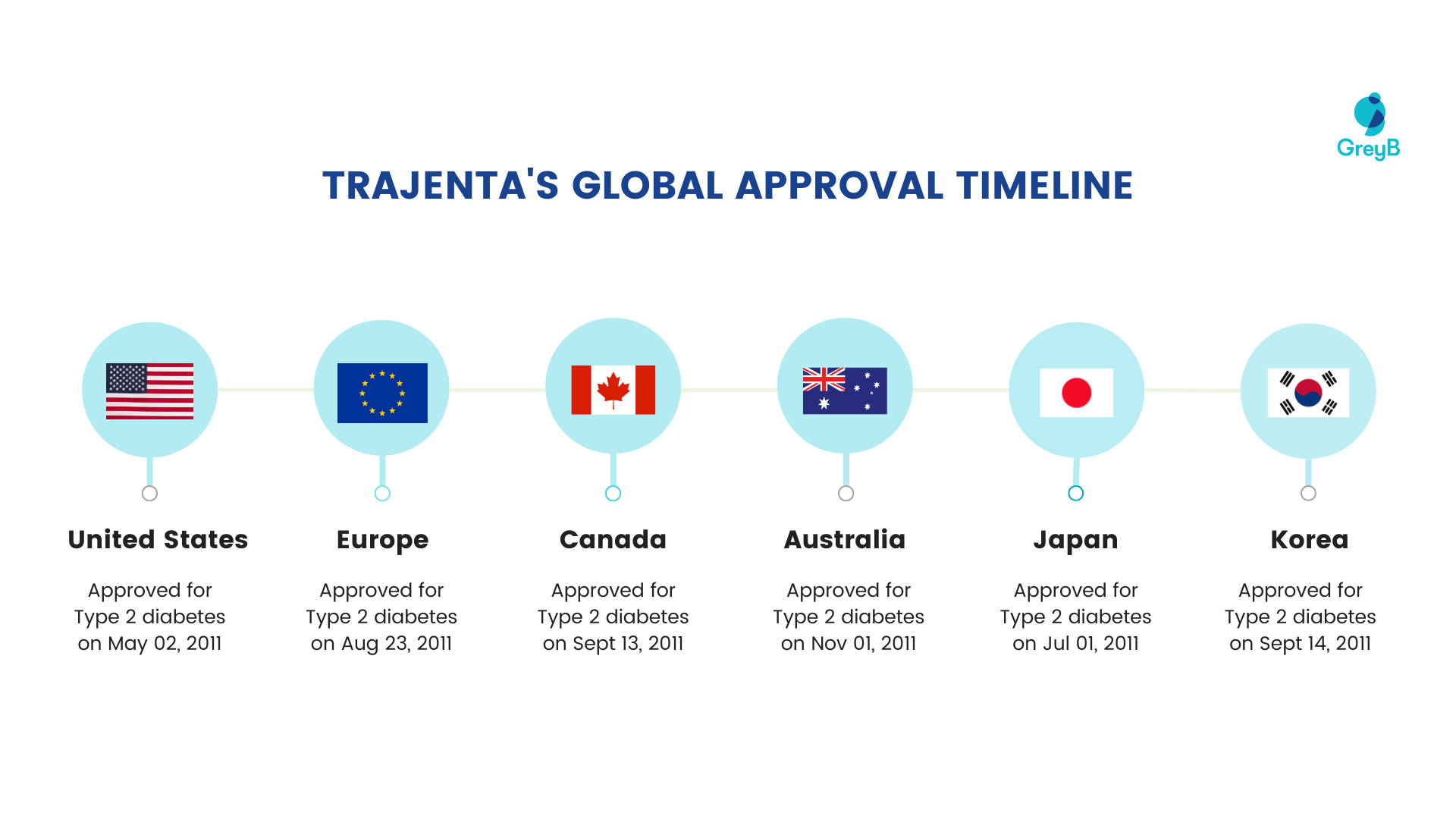
The drug was first approved by the US FDA in May 2011 for treating type 2 diabetes. It was later approved by other regulatory bodies in Europe, Australia, Canada, Japan, and Korea in the same year (2011).
Here is the timeline for which Trajenta was approved by different countries:
Just how important is Trajenta® for Boehringer Ingelheim?
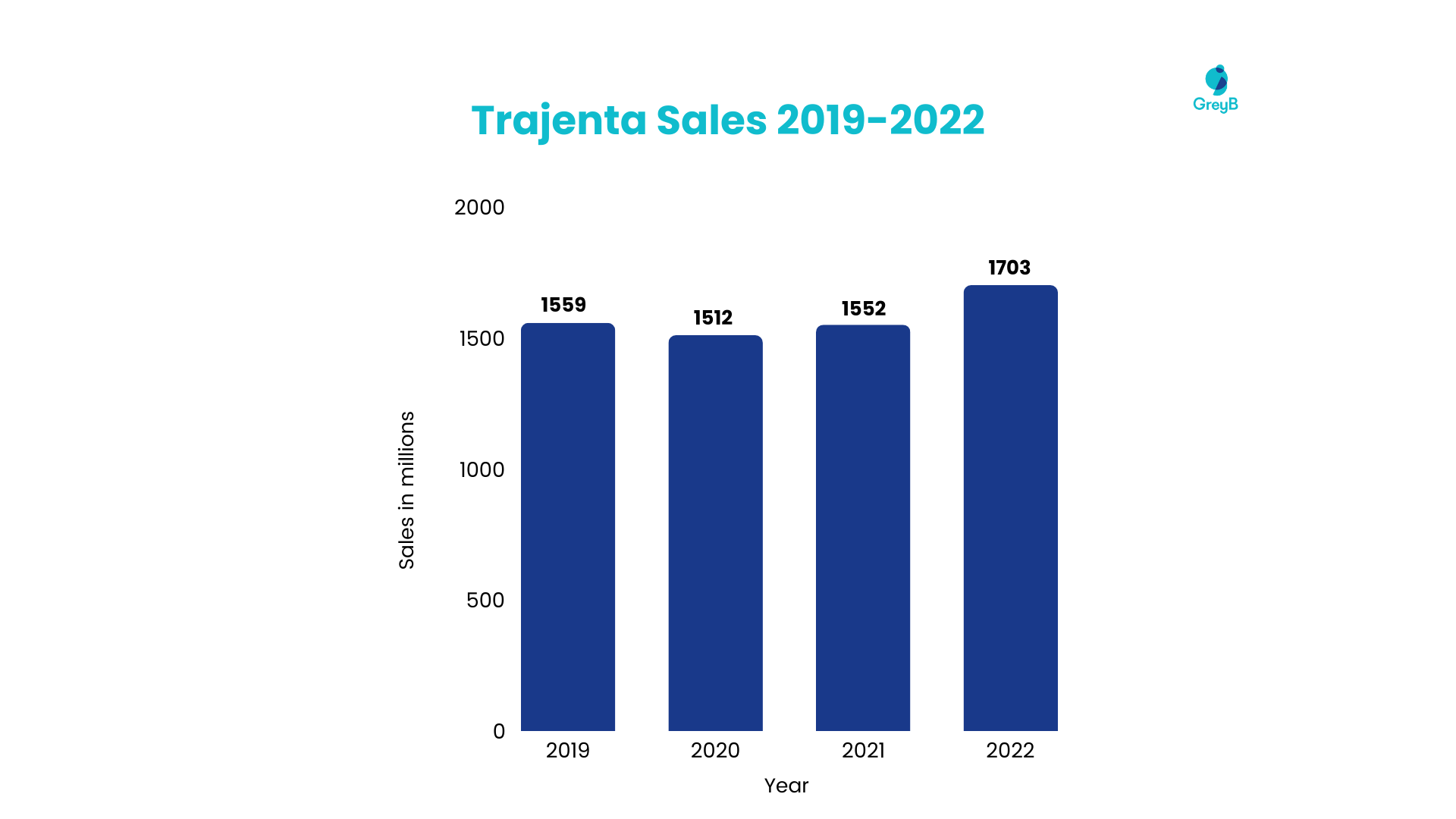
Linagliptin has secured substantial sales, positioning itself as a go-to solution for many competitor companies for diabetes. The driving force behind its robust market presence is reinforcing its molecule patent. However, Linagliptin’s molecule patent is set to expire in 2025. This information will significantly shift, opening doors to increased accessibility and affordability for patients.
Like every drug company, Boehringer has strategically filed multiple patents around the drug to protect its position and investment, forming a patent thicket.
Here’s how it looks –
| Indications | Type 2 Diabetes/Glycemic Control (Monotherapy) | Type 2 Diabetes/Glycemic Control combination with metformin; sulfonylurea (with or without metformin); metformin and empagliflozin; insulin (with or without metformin) | Conditions related to Type 2 Diabetes(Diabetic wound, sepsis, nephropathy and reduced body weight) |
| Composition of Matter | US7407955B2 Expires on Nov 02, 2025 EP2849754B1 Expires on May 13, 2033 | ||
| Indication/ Method of Treatment | US20220160717A1, US20160015714A1 Under Application Stage | US11666590B2 Expires on Apr 03, 2034 EP4119136A1,EP4209210A1EP2996724A1 Under Application Stage | US9186392B2, Expires on May 05, 2031 EP2854824A1Under Application Stage |
| Dosage Regimen | US10034877B2, Expires on Feb 05, 2030 US9149478B2 Expires on Jan 06, 2033 AU2017272209B2 Expires on Nov 15, 2031 US20210205316A1, US20210379073A1, US20120122776A1 Under Application Stage | US8673927B2, Expires on Nov 04, 2027, US8846695B2, Expires on Dec 04, 2030, US10406172B2, Expires on Jun 15, 2030, US9713618B2, Expires on May 05, 2031, US10092571B2, Expires on Nov 26, 2030, PH12011501619B1 Expires on Aug 12, 2031 US20230381188A1, US20220088023A1 Under Application stage | CA3065586C, Expires on Nov 15, 2031 |
| Formulation | US11033552B2 Expires on Nov 04, 2027 US9415016B2 Expires on Oct 02, 2029 US20220331326A1,US20170095423A1,OA15517A,GC0011427AUnder Application stage | US9555001B2 Expires on Sep 06, 2033 US8513264B2 Expires on Sep 08, 2029 PH12011501611B1 Expires on Aug 11, 2031 US20220105043A1, US20160106677A1, US20140079781A1, US20130172244A1, TW201249480A Under Application stage | – |
| Manufacturing | US8883805B2, Expires on May 26, 2026 US9815837B2, Expires on May 04, 2027 US10253026B2 Expires onJun 24, 2036 | – | |
As top blockbuster drugs approach patent expiration, strategic ANDA filings are critical to secure early mover advantage. Download the Pharsight Digester report now for a deep dive into the patent strategies of the top 5 blockbuster drugs nearing expiration.
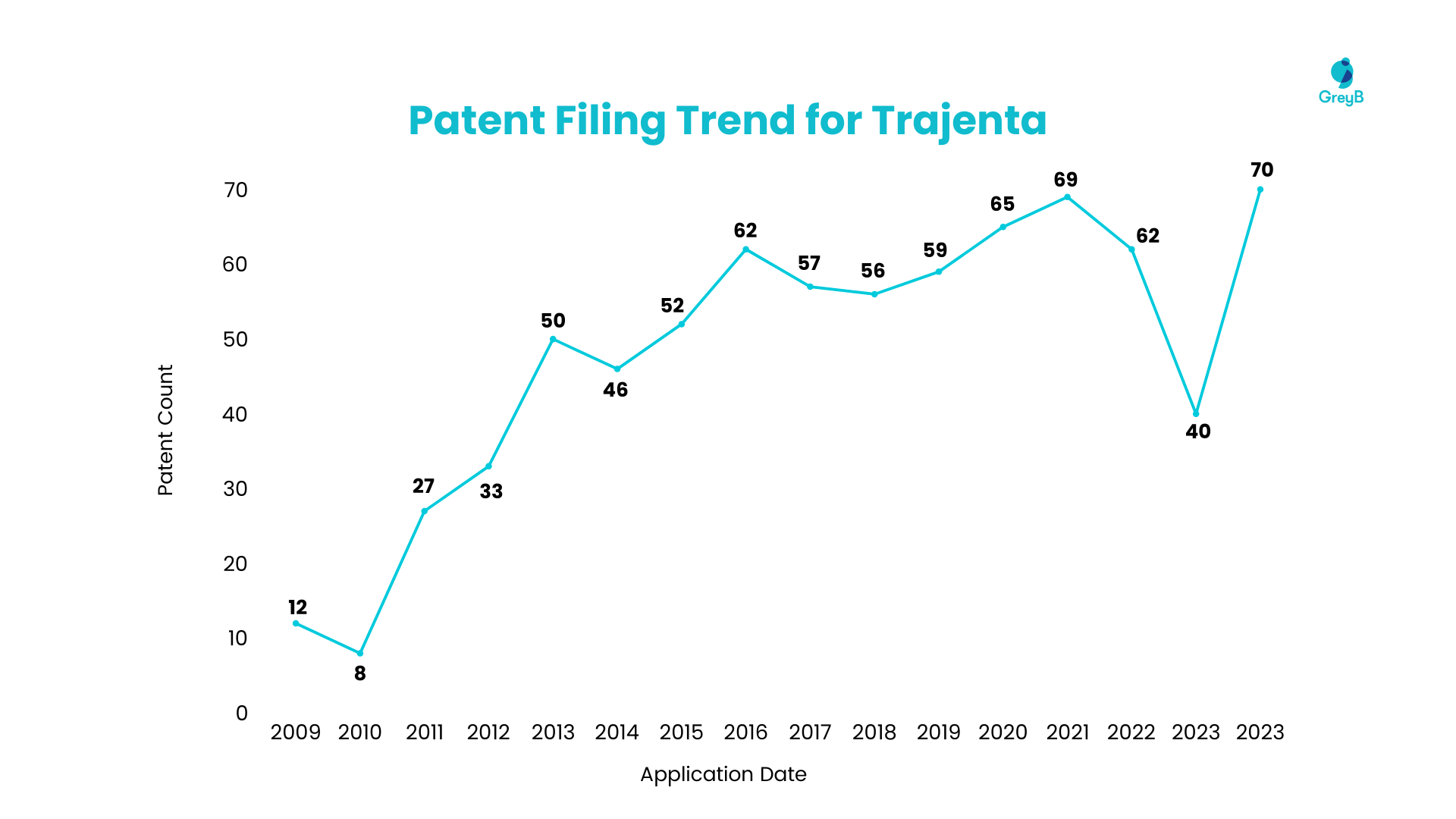
Patent Filing Trend for Linagliptin
The number of patents for Linagliptin began to rise in 2008, reaching a peak in 2013, with approximately 50 patents filed in a single year. Starting in 2018, patent filings have seen a substantial annual increase compared to previous years. The upward trend in Linagliptin’s patent filings became more pronounced in 2011, reaching its peak in 2021 with over 60 patents filed, indicating significant interest from pharmaceutical companies in this drug.
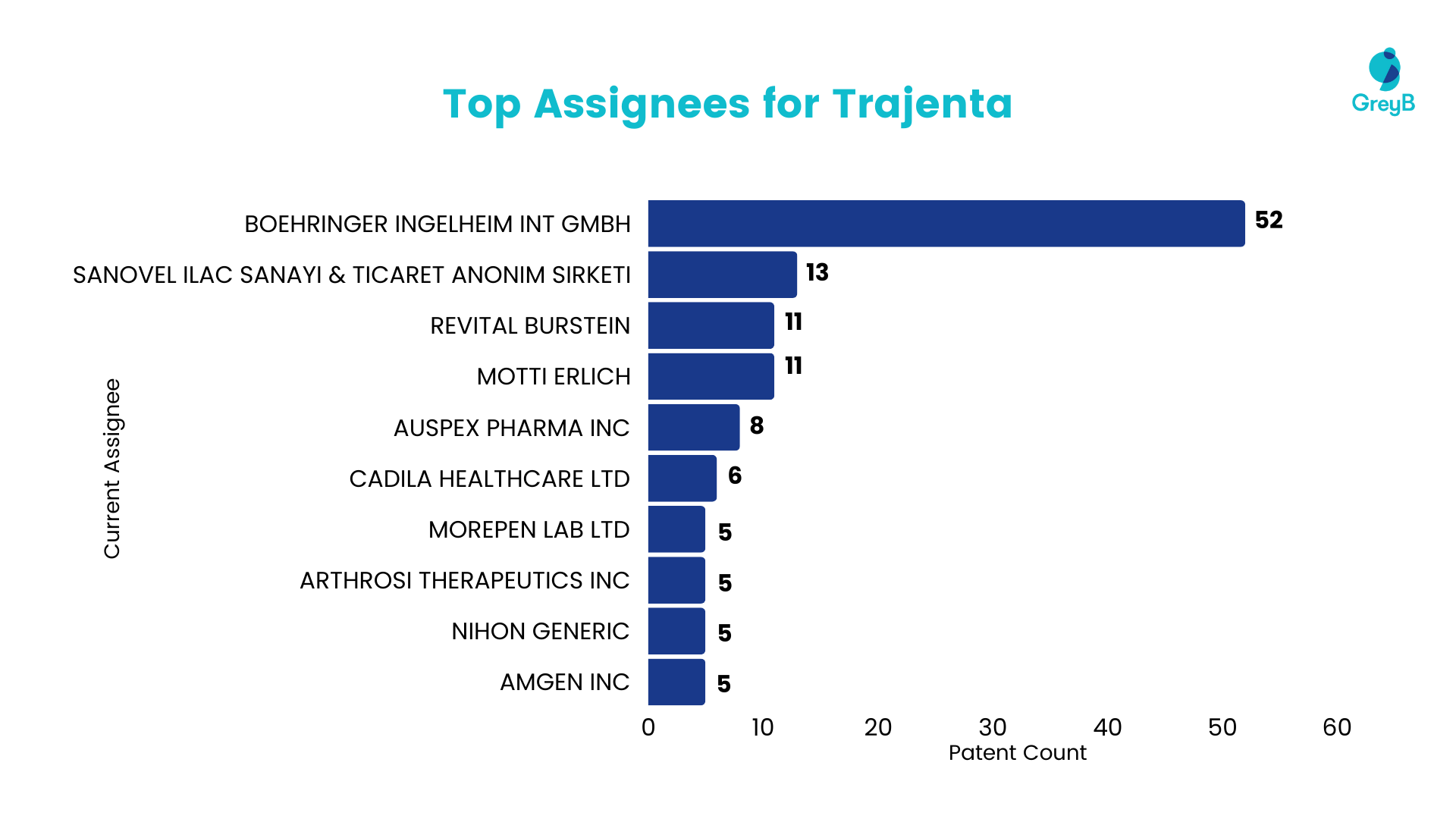
Top Assignees for Linagliptin
The company actively filing patents for Linagliptin is Boehringer (Originator Company), which has over 50 patents. Apart from Boehringer, Sanovel ilac and Revital Burstein have filed over 10 patents each. The active involvement of companies in Linagliptin patent filings underscores a collective industry interest in this particular drug.
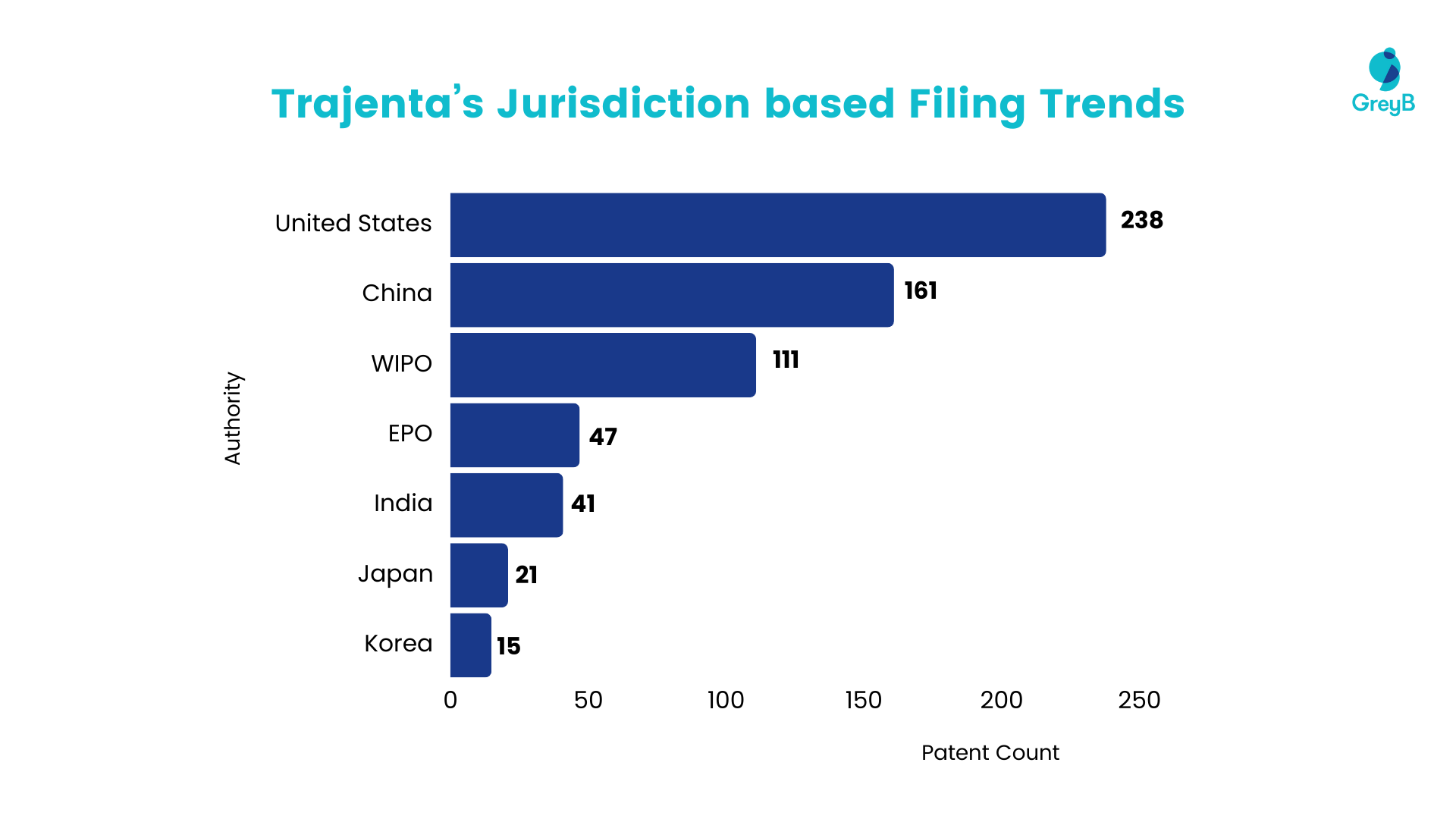
Filing Trend Based on Jurisdiction
The data presented below reveals a distinct pattern in patent filings, indicating a notable concentration of activity in the United States and China. A detailed examination of the figures demonstrates substantial market interest, with companies submitting a total of over 200 patents in the United States and exceeding 150 patents in China.
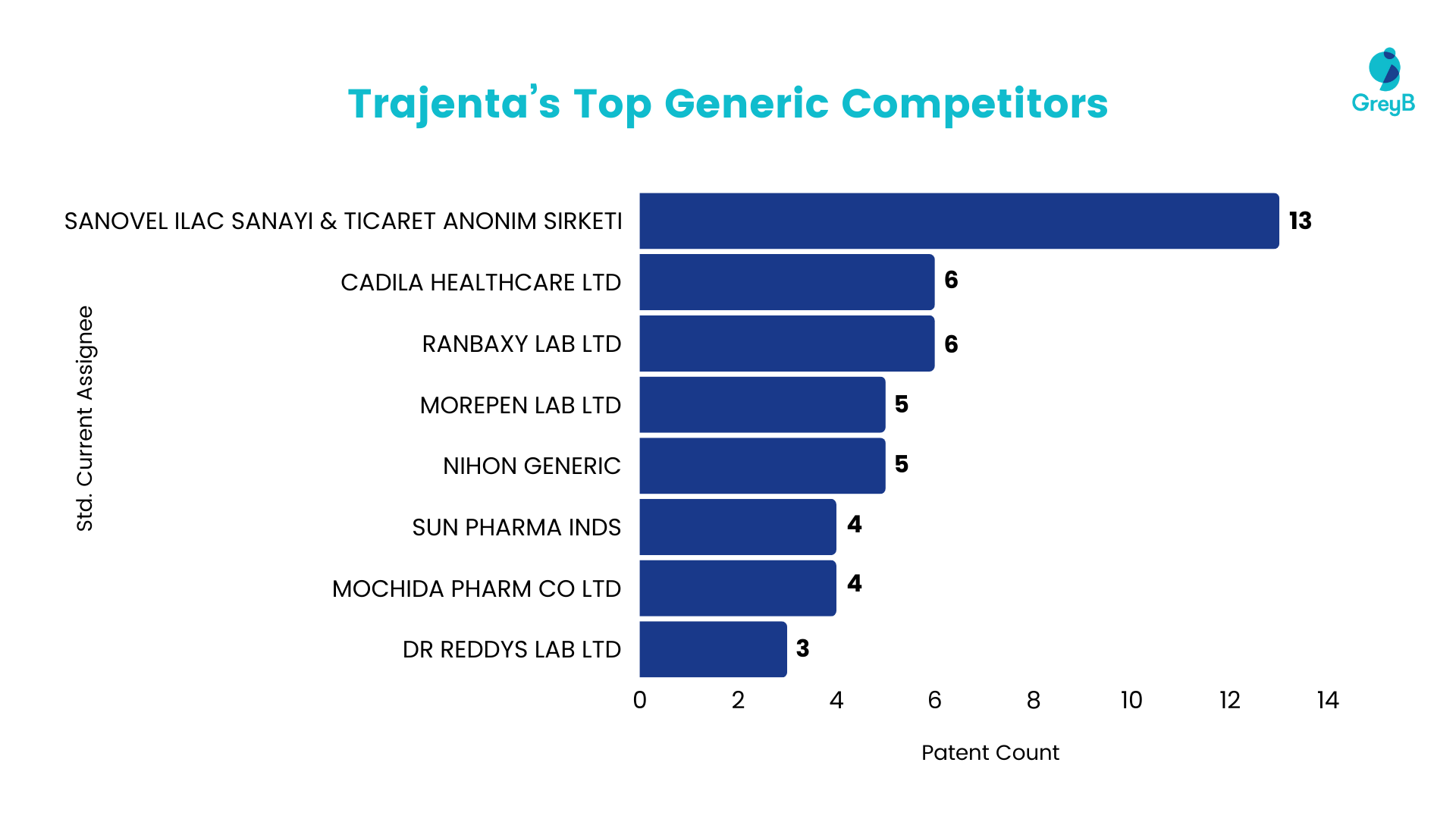
Generic Competitors
Linagliptin has attracted attention from generic drug manufacturers, as evidenced by the significant number of patents filed. This trend suggests a potential intention to introduce a generic version of the drug in the future. Sanovel ilac stands out as the most active participant in patent filings for this drug, followed by other generic companies such as Cadila, Ranbaxy (now Sun Pharma), Morepen Lab, and Nihon Generic, all indicating their interest in Linagliptin through patent applications.
Entry of Generic Version of Trajenta: A Prediction
The expiration of a key patent for Linagliptin will allow generic manufacturers to introduce their versions, offering cost-effective options for consumers. However, it’s crucial to note that, molecule patent will expire only in the US in 2025, while it will remain active in Europe until May 2033, preventing entry into the European market.
Moreover, the entry of generics into the US market will still encounter challenges due to the active formulation patent set to expire in November 2027. Although applications for generic versions can be filed with regulatory bodies, products cannot be launched until formulation patents expire.
In summary, while Linagliptin is nearing entry into the generic market, a thorough examination of other patents related to this drug is essential before market entry.
Elixir helps you stay vigilant and monitor activities related to the patents, giving you a headstart in the generic market.
Be the first one to grab the largest share of the generic market. Try Elixir today!
Authored By – Shadab Riyaz, Life Sciences
Edited By – Ridhima Mahajan, Marketing
Also Read – Understanding the Farxiga®/Forxiga® Patent Thicket: What’s Changing in 2025









